INTRODUCTION
MATERIALS AND METHODS
Patients
Demographic and clinical characteristics of three groups
Skin prick test
Lung function test
Subjective and objective assessment of CRS
Statistical analysis
RESULTS
Patients’ characteristics
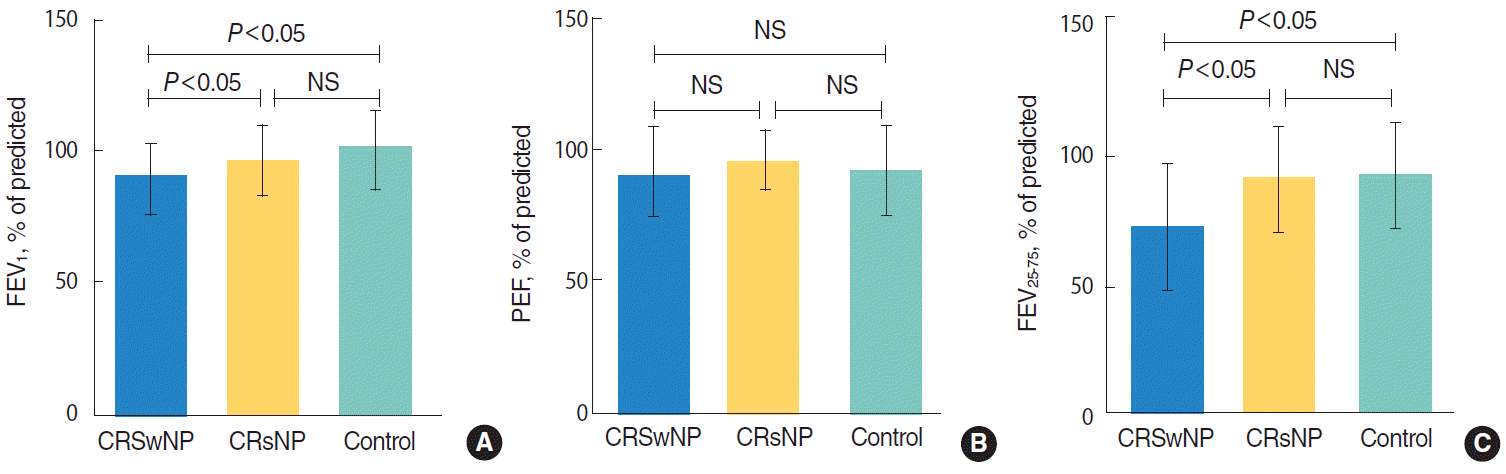 | Fig. 1.Forced expiratory volume in 1 second (FEV1), peak expiratory flow (PEF), forced expiratory flow between 25% and 75% of forced vital capacity (FEF25-75) in CRSwNP, CRSsNP and control group. (A, C) FEV1 and FEF25-75 were significantly lower. (B) PEF was no significantly different among three groups. CRSwNP, chronic rhinosinusitis with nasal polyps; CRSsNP, chronic rhinosinusitis without nasal polyps; NS, no significance. |
Table 1.
CRSwNP, chronic rhinosinusitis with nasal polyps; CRSsNP, chronic rhinosinusitis without nasal polyps; CRS, chronic rhinosinusitis; PBEC, peripheral blood eosinophil count; LMS, Lund-Mackay score; FEV1, forced expiratory volume in 1 second; PEF, peak expiratory flow; FEF25–75, forced expiratory flow between 25% and 75% of forced vital capacity.
Correlation analysis of FEV1, PEF, FEF25-75 in CRSwNP group
Table 2.
| Variable | PBEC | DD | SSN | VAS | SNOT-20 | LMS | LKS |
|---|---|---|---|---|---|---|---|
| FEV1 | –0.348* | –0.344* | –0.078 | –0.127 | –0.033 | –0.067 | –0.130 |
| PEF | 0.042 | –0.274 | 0.014 | –0.143 | –0.128 | –0.154 | –0.176 |
| FEF25-75 | –0.149 | –0.237 | –0.056 | –0.128 | –0.180 | –0.070 | –0.135 |
FEV1, forced expiratory volume in 1 second; PEF, peak expiratory flow; FEF25–75, forced expiratory flow between 25% and 75% of forced vital capacity; CRSwNP, chronic rhinosinusitis with nasal polyps; PBEC, peripheral blood eosinophil count; DD, duration of disease; SSN, sinus surgery number; VAS, visual analogue scale; SONT-20, 20-item Sino-Nasal Outcome Test; LMS, Lund-Mackay score; LKS, Lund-Kennedy endoscopic score.
Correlation analysis of FEV1, PEF, FEF25-75 in CRSsNP group
Table 3.
| Variable | PBEC | DD | SSN | VAS | SNOT-20 | LMS | LKS |
|---|---|---|---|---|---|---|---|
| FEV1 | –0.068 | 0.388 | 0.352 | –0.030 | –0.150 | –0.196 | 0.048 |
| PEF | –0.108 | –0.108 | –0.182 | –0.034 | –0.092 | 0.218 | –0.095 |
| FEF25-75 | –0.156 | –0.010 | –0.134 | –0.490* | –0.478* | –0.103 | –0.062 |
FEV1, forced expiratory volume in 1 second; PEF, peak expiratory flow; FEF25–75, forced expiratory flow between 25% and 75% of forced vital capacity; CRSsNP, chronic rhinosinusitis without nasal polyps; PBEC, peripheral blood eosinophil count; DD, duration of disease; SSN, sinus surgery number; VAS, visual analogue scale; SNOT-20, 20-item Sino-Nasal Outcome Test; LMS, Lund-Mackay score; LKS, Lund-Kennedy endoscopic score.
A multiple linear regression model for change ratio of FEV1 before and after bronchial provocation or dilation test in CRSwNP group
Table 4.
| Variable | B | SE | β | P-value |
|---|---|---|---|---|
| Constant | –2.459 | 5.347 | ||
| Age | 0.093 | 0.069 | 0.195 | 0.184 |
| PBEC | 4.439 | 1.544 | 0.403 | 0.006* |
| DD | 0.007 | 0.007 | 0.158 | 0.359 |
| SSN | –0.715 | 1.176 | –0.109 | 0.546 |
| VAS | 0.065 | 0.147 | 0.100 | 0.662 |
| SNOT-20 | 0.011 | 0.234 | 0.009 | 0.961 |
| LMS | 0.211 | 0.145 | 0.243 | 0.153 |
| LKS | –0.067 | 0.254 | –0.047 | 0.795 |
CRSwNP, chronic rhinosinusitis with nasal polyps; FEV1, forced expiratory volume in 1 second; SE, standard error; PBEC, peripheral blood eosinophil count; DD, duration of disease; SSN, sinus surgery number; VAS, visual analogue scale; SNOT-20, 20-item Sino-Nasal Outcome Test; LMS, Lund-Mackay score; LKS, Lund-Kennedy endoscopic score.
A multiple linear regression model for change ratio of FEV1 before and after bronchial provocation or dilation test in CRSsNP group
Table 5.
CRSsNP, chronic rhinosinusitis without nasal polyps; FEV1, forced expiratory volume in 1 second; SE, standard error; PBEC, peripheral blood eosinophil count; DD, duration of disease; SSN, sinus surgery number; VAS, visual analogue scale; SNOT-20, 20-item Sino-Nasal Outcome Test; LMS, Lund-Mackay score; LKS, Lund-Kennedy endoscopic score.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download