INTRODUCTION
MATERIALS AND METHODS
Patient selection
18F-FDG PET/CT and CE CT
CE neck CT
Image analysis and information obtained
Gold standard for the final diagnosis
Table 1.
| Variable | No. (%) |
|---|---|
| No. of patients (center A:B:C:D) | 53 (62):14 (17):13 (15): 5 (6) |
| Sex (male:female) | 14 (17):71 (84) |
| Age (yr), median (range) | 54 (19–80) |
| CE PET/CT arm position (up:down) | 54 (64):31 (37) |
| Clinical follow-up methods to confirm | |
| Tg+US | 23 (27) |
| Tg+US+RI | 14 (17) |
| Tg+US+RI+biopsy | 2 (2) |
| Tg+RI | 17 (20) |
| Tg | 29 (34) |
| Preoperative staging (N0:N1a:N1b) | |
| By neck CT | 62 (73): 5 (6): 18 (21) |
| By CE PET/CT | 57 (67): 9 (11): 19 (22) |
| Pathologic results at operation | |
| pT1:pT2:pT3:pT4 | 30 (35): 2 (2): 48 (57): 5 (6) |
| Tumor side (left:right:both) | 25 (29): 33 (39): 27 (32) |
| Final results of follow-up of lymph nodea) | |
| N0:N1a:N1b | 47 (55): 18 (21): 20 (24) |
| Central lymph node metastasis only, no. of patients (level) | 34 (38) |
| Lateral lymph node metastasis (ipsilateralb):bilateral:contralateral only) | 19:2:0 |
| Overall stage (I:II:III:IVA)c) | 25 (29): 0 (0):38 (45): 22 (26) |
CE PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography with contrast-enhancement; Tg, serum level of thyroglobulin; US, neck ultrasonography; RI, radioactive iodine whole body scan.
b) For tumors in both sides of thyroid lobes, metastatic lymph nodes in either sides of the neck were considered as ipsilateral metastasis. In analysis of ipsilateral metastasis, central lymph nodes (level VI and VII) were excepted. There was no case with lymph node metastasis to contralateral neck without ipsilateral side metastasis.
Statistical analysis
RESULTS
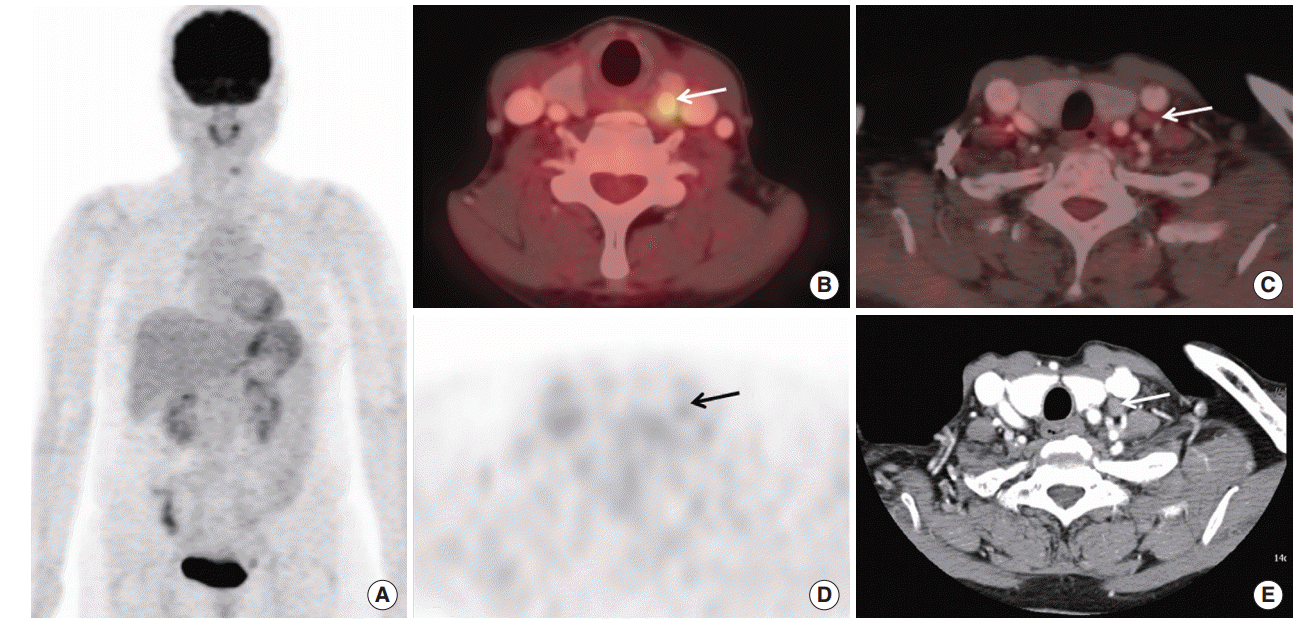 | Fig. 1.A case of different finding of 18F-fluorodeoxyglucose positron emission tomography/computed tomography with contrast-enhancement (CE PET/CT) and contrast-enhanced neck CT (neck CT) in a preoperative lymph node staging in a patient with thyroid cancer. A 56-year-old female patient underwent CE PET/CT and neck CT with contrast-enhancement before thyroid cancer operation: (A) maximum intensity image of CE PET/CT, (B, C) axial fusion images, (D) axial PET only image at the same level of C, and (E) axial image of neck CT. A calcified nodule with focal hypermetabolism with maximum standardized uptake value (SUVmax) 4.2 was found in left thyroid lobe which was proven to be papillary cancer by fine needle aspiration (arrow in B). Both of CE PET/CT and neck CT showed a lymph node adjacent to left carotid vessel (arrows in C-E). The long diameter by neck CT was about 1 cm (E), suggesting metastatic lymph node. However, FDG uptake was not high (arrows in D, SUVmax 1.4), suggesting benign feature. After operation, biopsy revealed benign lymph node. Also, clinical follow-up with radioactive iodine scan and neck ultrasonography (images not shown), there was no metastatic lymph node. |
Analysis by patients
Table 2.
| Variable | TP | FP | FN | TN | Sensitivity (%, CI) | Specificity (%, CI) | Accuracy (%) | PPV (%, CI) | NPV (%, CI) |
|---|---|---|---|---|---|---|---|---|---|
| By patients (N0, N1) | |||||||||
| Neck CT | 17 | 6 | 21 | 41 | 44.7 (28.6–61.7) | 87.2 (74.3–95.2) | 68.2 | 73.9 (51.6–89.8) | 66.1 (53.0–77.7) |
| CE PET/CT | 25 | 3 | 13 | 44 | 65.8 (48.7–80.4) | 93.6 (82.5–98.7) | 81.2 | 89.3 (71.8–97.7) | 77.2 (64.2–87.3) |
| P-value | 0.008a) | 0.375 | 0.078 | 0.055 | 0.001a) | ||||
| By ipsilateral levels | |||||||||
| I | |||||||||
| Neck CT | 1 | 0 | 0 | 84 | 100 (2.5–100) | 100 (95.7–100) | 100 | 100 (2.5–100) | 100 (95.7–100) |
| CE PET/CT | 0 | 0 | 1 | 84 | 0 (0–97.5) | 100 (95.7–100) | 98.8 | NA | 98.8 (93.6–99.9) |
| P-value | NA | NA | 0.998 | NA | NA | ||||
| II | |||||||||
| Neck CT | 6 | 3 | 6 | 70 | 50 (21.1–78.9) | 95.9 (88.5–99.1) | 89.4 | 66.7 (29.9–92.5) | 92.1 (83.6–97.2) |
| CE PET/CT | 9 | 1 | 3 | 72 | 75 (42.8–94.5) | 98.6 (92.6–100) | 95.29 | 90 (55.5–99.8) | 96 (88.9–99.2) |
| P-value | 0.25 | 0.5 | 0.249 | 0.072 | 0.070 | ||||
| III | |||||||||
| Neck CT | 11 | 0 | 8 | 66 | 57.9 (33.5–79.8) | 100 (94.5–100) | 90.7 | 100 (71.5–100) | 89.3 (79.8–95.2) |
| CE PET/CT | 13 | 1 | 6 | 65 | 68.4 (43.5–87.4) | 98.5 (91.8–100) | 91.8 | 92.9 (66.1–99.8) | 91.6 (82.5–96.8) |
| P-value | 0.688 | 1.0 | 1.0 | 0.301 | 0.437 | ||||
| IV | |||||||||
| Neck CT | 6 | 2 | 9 | 68 | 40.0 (16.3–67.7) | 97.1 (90.1–99.7) | 87.1 | 75.0 (34.9–96.8) | 88.3 (79.0–94.5) |
| CE PET/CT | 13 | 4 | 2 | 66 | 86.7 (59.5–98.3) | 94.4 (86.0–98.4) | 93.0 | 76.5 (50.1–93.2) | 97.1 (89.8–99.6) |
| P-value | 0.016a) | 0.620 | 0.367 | 0.911 | 0.006a) | ||||
| V | |||||||||
| Neck CT | 0 | 1 | 2 | 82 | 0 (0–84.2) | 98.8 (93.5–100) | 96.5 | 0 (0–97.5) | 97.6 (91.7–99.7) |
| CE PET/CT | 1 | 0 | 1 | 83 | 50 (1.3–98.7) | 100 (95.7–100) | 98.8 | 100 (2.5–100) | 98.8 (93.5–100) |
| P-value | 1.0 | 1.0 | 0.614 | 0.157 | 0.309 | ||||
| VI (central) | |||||||||
| Neck CT | 10 | 3 | 24 | 48 | 29.4 (15.1–47.5) | 94.1 (83.8–98.8) | 68.2 | 76.9 (46.2–95.0) | 66.7 (54.6–77.3) |
| CE PET/CT | 18 | 3 | 16 | 48 | 52.9 (35.1–70.2) | 94.1 (83.8–98.8) | 77.7 | 85.7 (63.7–97.0) | 75.0 (62.6–85.0) |
| P-value | 0.008a) | 1.0 | 0.227 | 0.312 | 0.004a) | ||||
| VII | |||||||||
| Neck CT | 3 | 2 | 1 | 79 | 75 (19.4–99.4) | 97.5 (91.4–99.7) | 96.5 | 60 (14.7–94.7) | 98.8 (93.2–99.9) |
| CE PET/CT | 3 | 2 | 1 | 79 | 75 (19.4–99.4) | 97.5 (91.4–99.7) | 96.5 | 60 (14.7–94.7) | 98.8 (93.2–99.9) |
| P-value | NA | 1.0 | 0.678 | 1.0 | 1.0 | ||||
| By contralateral levelsb) | |||||||||
| I | |||||||||
| Neck CT | 0 | 0 | 0 | 58 | NA | 100 (93.8–100) | 100 | NA | 100 (93.8–100) |
| CE PET/CT | 0 | 1 | 0 | 57 | NA | 98.3 (90.8–100) | 98.3 | 0 (0–97.5) | 100 (93.7–100) |
| P-value | NA | 1.0 | 0.989 | NA | NA | ||||
| II | |||||||||
| Neck CT | 0 | 1 | 1 | 56 | 0 (0–97.5) | 98.3 (90.6–100) | 95.6 | 0 (0–97.5) | 98.3 (90.6–100) |
| CE PET/CT | 0 | 0 | 1 | 57 | 0 (0–97.5) | 100 (93.7–100) | 98.3 | NA | 98.3 (90.8–100) |
| P-value | NA | 1.0 | 0.760 | NA | NA | ||||
| III | |||||||||
| Neck CT | 0 | 0 | 0 | 58 | NA | 100 (93.8–100) | 100 | NA | 100 (93.8–100) |
| CE PET/CT | 0 | 0 | 0 | 58 | NA | 100 (93.8–100) | 100 | NA | 100 (93.8–100) |
| P-value | NA | NA | NA | NA | NA | ||||
| IV | |||||||||
| Neck CT | 1 | 1 | 0 | 56 | 100 (2.5–100) | 98.25 (90.6–100) | 98.3 | 50 (1.3–98.7) | 100 (93.6–100) |
| CE PET/CT | 1 | 0 | 0 | 57 | 100 (2.5–100) | 100 (93.7–100) | 100 | 100 (2.5–100) | 100 (93.7–100) |
| P-value | NA | 1.0 | 0.989 | 0.18 | NA | ||||
| V | |||||||||
| Neck CT | 0 | 0 | 0 | 58 | NA | 100 (93.8–100) | 100 | NA | 100 (93.84–100) |
| CE PET/CT | 0 | 0 | 0 | 58 | NA | 100 (93.8–100) | 100 | NA | 100 (93.8–100) |
| P-value | NA | NA | NA | NA | NA | ||||
| By neck side | |||||||||
| Ipsilateral sidec) | |||||||||
| Neck CT | 13 | 2 | 6 | 64 | 68.4 (43.5–87.4) | 97.0 (89.5–99.6) | 90.6 | 86.7 (59.5–98.3) | 91.4 (82.3–96.8) |
| CE PET/CT | 16 | 2 | 3 | 64 | 84.2 (60.4–96.6) | 97.0 (89.5–99.6) | 94.1 | 88.9 (65.3–98.6) | 95.5 (87.5–99.1) |
| P-value | 0.25 | 1.0 | 0.569 | 0.838 | 0.078 | ||||
| Contralateral sided) | |||||||||
| Neck CT | 1 | 1 | 1 | 55 | 50.0 (1.4–98.7) | 98.2 (90.5–99.9) | 96.6 | 50.0 (1.4–98.7) | 98.21 (90.5–99.9) |
| CE PET/CT | 1 | 1 | 1 | 55 | 50.0 (1.4–98.7) | 98.2 (90.5–99.9) | 96.6 | 50.0 (1.4–98.7) | 98.21 (90.5–99.9) |
| P-value | NA | 1.0 | 0.608 | 1.0 | 1.0 |
Analysis of each cervical level: contralateral or ipsilateral
Analysis by number of cervical levels involved
Analysis of clinical factors affecting the accuracy of CE PET/CT and neck CT
Table 3.
| Variable |
CE PET/CT staging |
Neck CT |
||||
|---|---|---|---|---|---|---|
| Correct | Incorrect | P-value | Correct | Incorrect | P-value | |
| Age (yr) | 53.2±10.2 | 50.2±10.2 | 0.331 | 53.5±12.6 | 50.26±9.85 | 0.236 |
| Sex (male:female) | 14:52 | 2:17 | 0.506 | 13:45 | 3:24 | 0.251 |
| PET/CT machine model (A:B:C:D:E) | 33:8:13:7:5 | 11:1:4:3:0 | 0.579 | 32:8:9:5:4 | 12:1:8:5:1 | 0.209 |
| PET arm position (up:down) | 42:24 | 12:7 | 0.816 | 36:22 | 18:9 | 0.867 |
| Tumor size (cm) | 0.8 (0.2–5.0) | 0.9 (0.4–2.8) | 0.321 | 0.8 (0.2–5.0) | 1.1 (0.3–3.8) | 0.062 |
| Extra-thyroidal extension (none:micro:macro) | 26:33:7 | 6:13:0 | 0.861 | 23:29:6 | 9:17:1 | 0.982 |
| Tumor location (unilateral:bilateral) | 49:17 | 9:10 | 0.053 | 42:16 | 16:11 | 0.336 |
| No. of primary tumors | 1 (1–7) | 2 (1–5) | 0.016a) | 1 (1–7) | 2 (1–5) | 0.136a) |
| No. of patients with multiple tumors (1:2:3:4:5:6 tumors) | 44:16:3:2:0:1 | 6:11:1:1:0:0 | 0.099b) | 38:14:3:2:0:1 | 12:13:1:1:0:0 | 0.441b) |
| Primary tumor SUVmax | 3.9 (1.5–49.5) | 6.0 (2.2–50.7) | 0.087 | 3.9 (1.5–48.5) | 5.5 (1.7–50.68) | 0.199 |
| Interval from scan to operation (day) | 18.5 (1–78) | 22 (0–62) | 0.804 | 18.5 (1–78) | 22 (0–64) | 0.610 |
| Institution (A:B:C:D) | 41:11:9:5 | 12:3:4:0 | 0.58 | 10:6:8:4 | 13:8:5:1 | 0.110 |
Values are presented as mean±standard deviation (age) or median with range (tumor size, number of primary tumors, SUVmax, and interval). For analysis, we used groups of “correct”/“incorrect” cases of each modalities based on the final results which were determined by surgical biopsy and clinical follow-up till postoperative 6 months. For example, when CE PET/CT successfully staged the LN (N0, N1a, and N1b) in some patients, they were categorized as CE PET/CT correct cases. PET, positron emission tomography; CT, computed tomography; CE PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography with contrast-enhancement; SUVmax, maximum standardized uptake value.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download