INTRODUCTION
MATERIALS AND METHODS
Preparation of ASD
Cell culture and reagents
Cell viability assay
Real-time reverse transcriptase polymerase chain reaction
Table 1.
Western blot analysis
Animal maintenance and experimental design
Histological evaluation
Statistical analysis
RESULTS
Cell viability assay
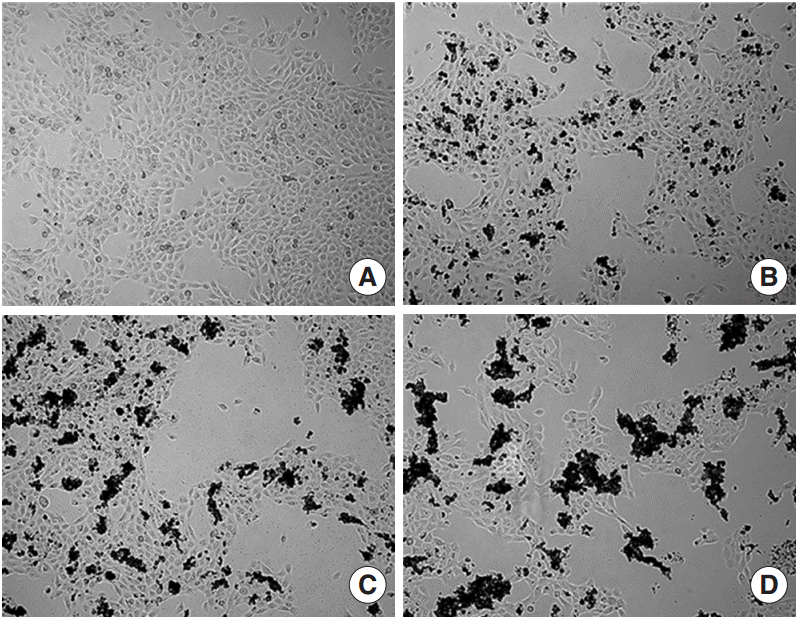 | Fig. 1.Microscopic images of human middle ear epithelial cell morphology after exposure to Asia sand dust (ASD) for 24 hours. (A) The control group showed 80% of confluence and unified morphology. In contrast, the ASD-treated groups (B, 100 μg/mL; C, 200 μg/mL; and D, 400 μg/mL) demonstrated ASD particles on the cells and many cells were detached from the culture plate in the ASD groups, compared to what was observed in the control group. |
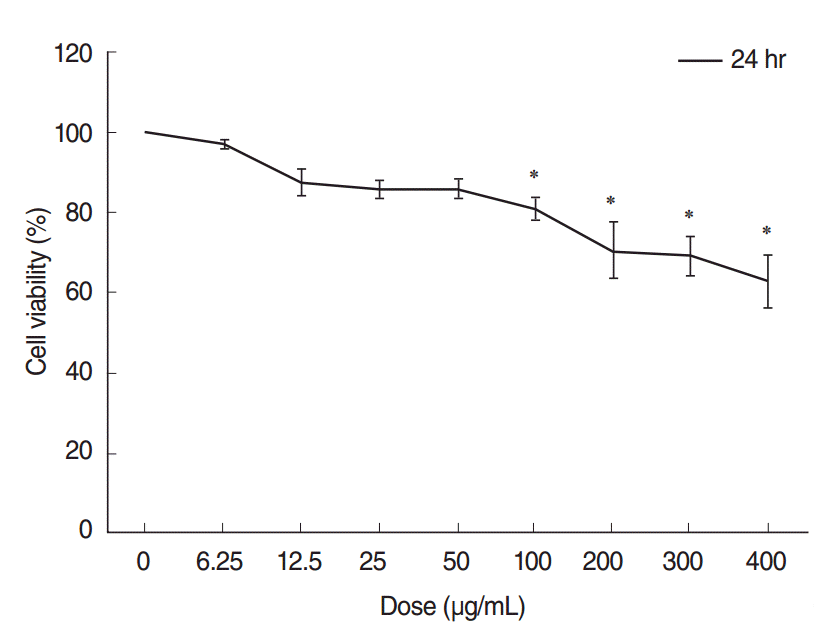 | Fig. 2.Cytotoxicity testing of human middle ear epithelial cells (HMEECs) exposed to Asia sand dust (ASD). Cells were treated with the indicated concentrations of ASD for 24 hours. Cell viability was calculated as the percentage of the viability of the control. HMEEC viability decreased in a dose dependent manner (0–400 μg/mL) when treated with ASD. Especially, administration of 400 μg/mL of ASD resulted in approximately 60% of viability compared to that of the control group. The results presented in the graph are from three independent experiments; the error bars indicate mean±SD. *P<0.05 compared to the control by analysis of variance. |
Real-time PCR analysis
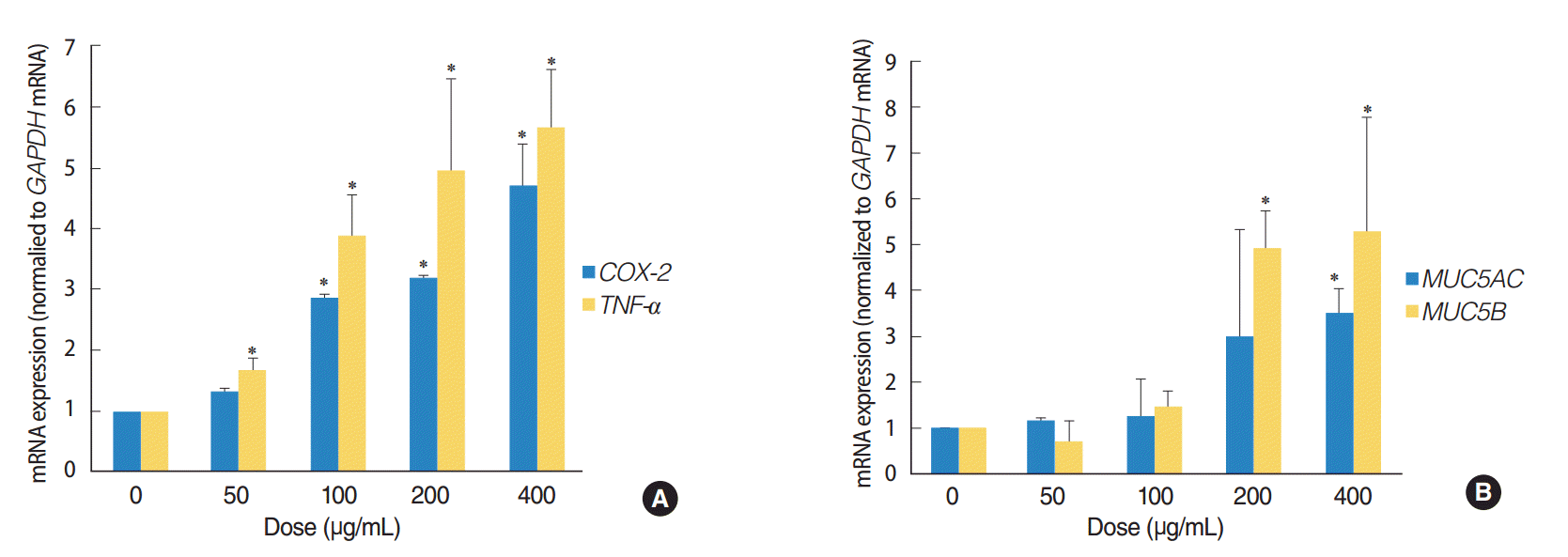 | Fig. 3.Asia sand dust (ASD) stimulates the expression of the inflammation and mucin production genes in human middle ear epithelial cells (HMEECs). HMEECs treated with ASD for 24 hours were subjected to quantitative real-time polymerase chain reaction analysis. HMEECs displayed an increase in inflammatory response and mucin gene expression upon stimulation with ASD in a dose-dependent manner. (A) TNF-a and COX-2 gene expressions were elevated significantly after treatment with ASD at concentrations greater than 100 μg/mL. (B) Treatment with ASD at concentrations greater than 200 μg/mL resulted in a significant increase in MUC5AC and MUC5B gene expressions. The error bars indicate mean±SD of multiple repetitive experiments. *P<0.05 compared to the control, determined by analysis of variance. |
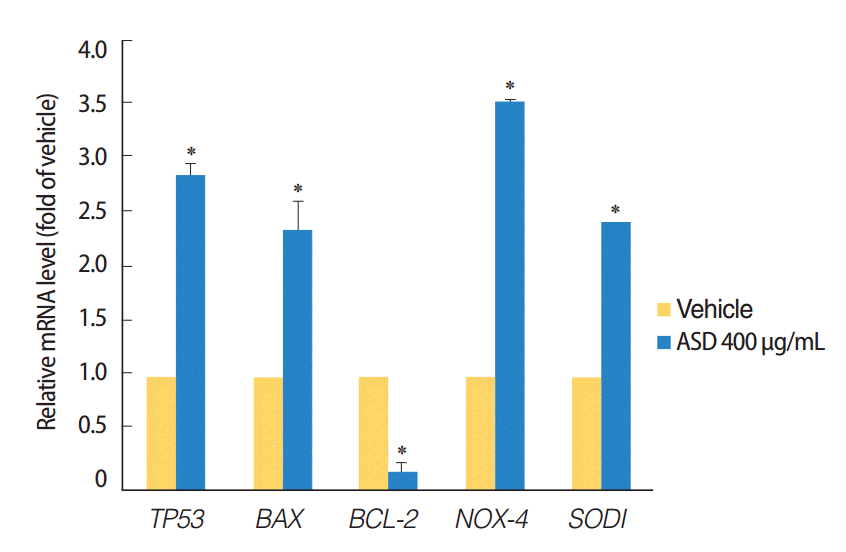 | Fig. 4.Expression levels of the apoptosis and reactive oxygen species production marker genes were investigated. The treatment of Asia sand dust (ASD) (400 μg/mL) for 24 hours resulted in the elevation of TP53 and BAX gene expressions. In contrast, the mRNA levels of the antiapoptotic gene BCL-2 were significantly down-regulated when treated with 400 μg/mL of ASD. ASD also induced an increase of NOX4 and SOD1 gene expressions in human middle ear epithelial cells. The error bars indicate mean±SD of multiple repetitive experiments; *P<0.05 compared to the control by analysis of variance. |
Western blot analysis
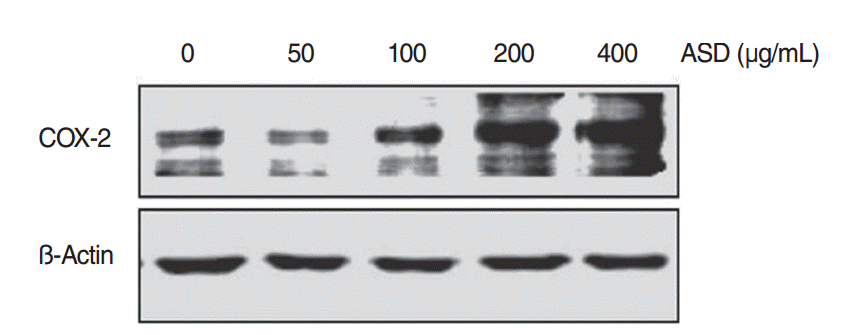 | Fig. 5.The levels of COX-2 protein increased when exposed to Asia sand dust (ASD) for 24 hours. Human middle ear epithelial cells were treated with ASD for 24 hours, at various concentrations (0, 50, 100, 200, or 400 μg/mL) and western blot analysis showed an increase of expression of COX-2 proteins in a dose-dependent manner. Results were obtained from three independent experiments. Beta-actin was used as the loading control. |
Histopathological examination of the middle ear
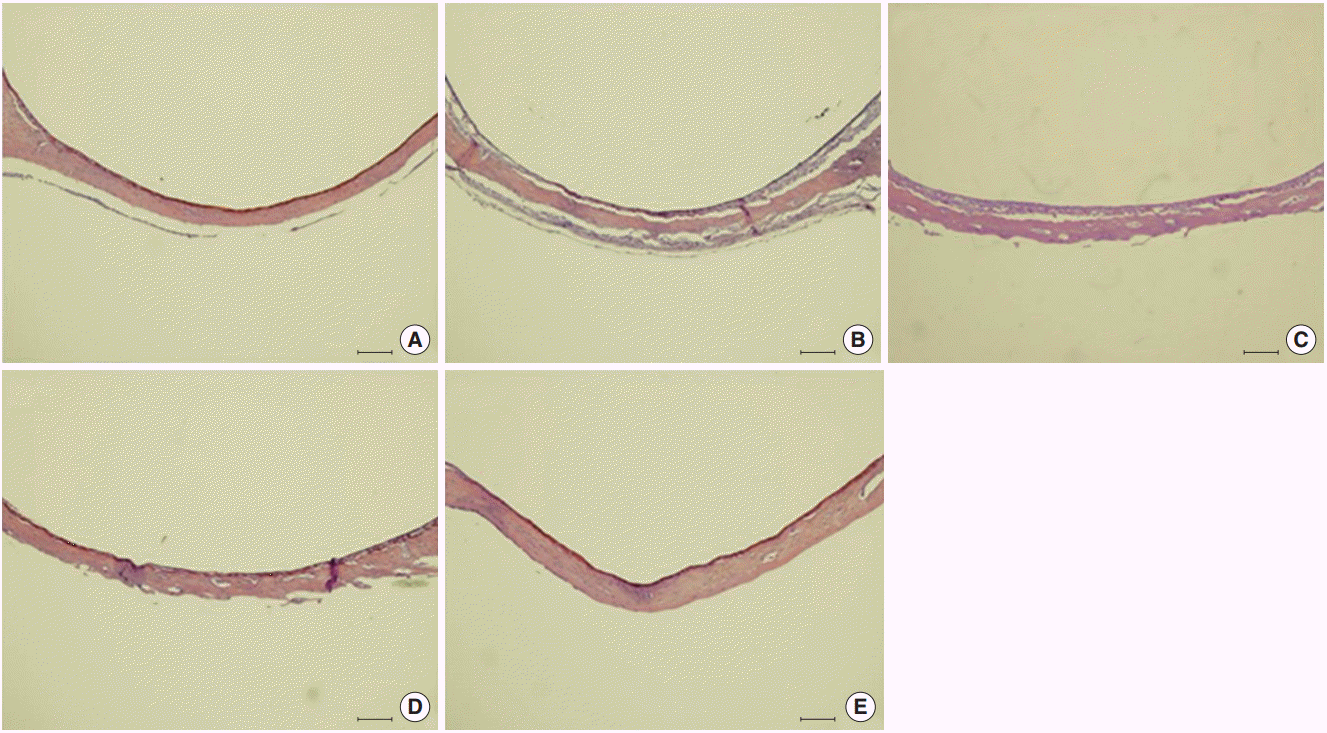 | Fig. 6.Histological changes in the middle ear are observed resulting from Asia sand dust (ASD) injection. Each panel displays representative images of a middle ear cavity at specifi c time points after ASD treatment. (A) shows the control animals (n=10) that received only phosphate buffered saline (vehicle). (B –E) display images of the middle ears taken at 1 (B), 3 (C), 5 (D), and 14 days (E) after ASD injection (each group, n=10). We observed a considerable thickening of the middle ear epithelium on days 1 and 3, which was gradually reduced by day 5. The thickened middle ear mucosa was normalized by day 14 (H&E, ×100, scale bar 100 μm). |
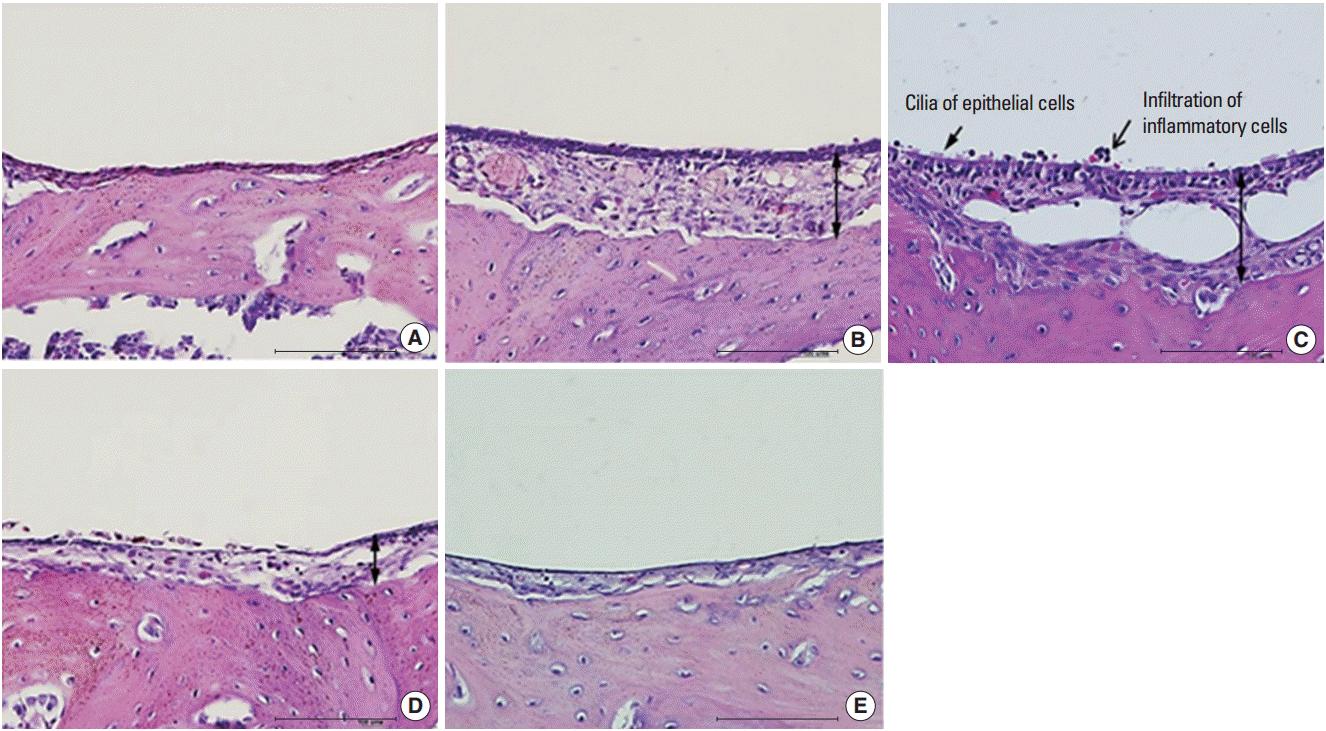 | Fig. 7.Histopathologic finding of otitis media demonstrated in Asia sand dust (ASD) injected rats. The 5 panels display representative images of the middle ear at a specific time point after ASD exposure: (A) control group, (B) day 1, (C) day 3, (D) day 5, and (E) day 14. Images demonstrate the pathological changes occurring as a result of ASD exposure. In particular, 3 days after ASD injection (C), we observed the appearance of cilia in the epithelium of the middle ear (arrow) and a considerable increase in the mucosal thickness (double-headed arrow) in the middle ear cavity. In addition, the middle ear cavity also displayed infiltration of the inflammatory cells (open arrow) (H&E, ×400, scale bar 100 μm) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download