Abstract
Despite widespread use of antibiotics and surgical procedures for treating peritonsillar abscess (PTA), symptoms of severe inflammation such as pain and trismus during treatment result in patient dissatisfaction. The goal of this study was to perform a systematic review and meta-analysis of the efficacy of systemic steroids on the clinical course of PTA. Two reviewers independently searched the databases (MEDLINE, Scopus, and the Cochrane Database) from inception to December 2014. Studies comparing systemic administration of steroids (steroid group) with placebo (placebo group), where the outcomes of interest were pain, body temperature, hospitalization, and oral intake during the posttreatment period, were included. Baseline study characteristics, study quality data, numbers of patients in the steroid and control groups, and outcomes were extracted. Sufficient data for meta-analysis were retrieved for 3 trials with a total of 153 patients. Pain-related parameters (patient-reported scores and trismus), body temperature, and dysphagia during the first 24 hours after treatment were significantly improved in the steroid group compared with placebo group. The discharge rate during the first 5 days of the posttreatment period was significantly higher in the steroid group than the control group. However, although more patients in the steroid group returned to normal activities and dietary intake at 24 hours after treatment, the differences between the groups were not significant and disappeared after 48 hours. In the treatment of PTA, systemic administration of steroids with antibiotics could reduce pain-related symptoms, as well as provide a benefit with respect to the clinical course. However, further trials with well-designed research methodologies should be conducted to confirm our results.
Peritonsillar abscesses (PTAs), formed in the potential space between the tonsillar capsule and superior constrictor muscle, are a complication of acute tonsillitis and the most common deep infection of the head and neck. They have an incidence of 30 per 100,000 with about 45,000 new cases annually. The annual cost of treating PTAs has been estimated at over $150 million [1]. Although most PTAs resolve with simple medical and surgical management, patients with PTAs experience odynophagia and trismus that prevent oral intake, thereby necessitating hospital admission for intravenous fluid replacement. Additionally, inadequately treated PTAs carry life-threatening complications—airway obstruction, abscess rupture and aspiration of pus, erosion or septic necrosis causing carotid sheath hemorrhage, and extension of the infection into the deep neck tissues or posterior mediastinum [2,3].
The treatment of PTAs requires both the selection of an appropriate antibiotic and an effective procedure to remove the pus collection. However, it is also important to focus on and resolve the severe inflammatory and spasmodic components of the disease because the spasm produces severe pain that prevents the mouth from opening fully, and hence the patients are unable to eat or drink [4]. Corticosteroids have been used to treat edema and inflammation in various otolaryngologic diseases [5]. In particular, corticosteroids inhibit transcription of proinflammatory mediators in human airway endothelial cells that cause pharyngeal inflammation and, ultimately, symptoms of pain [6,7]. Lamkin and Portt [8] reported that there was a striking degree of synergy between antibiotics and steroids in the treatment of various head and neck infections, and these regimens were very effective for the treatment of PTAs. Despite these promising findings, there is currently insufficient evidence in the literature to fully support the use of steroids in the treatment of PTAs. Considering that PTAs are commonly encountered by clinicians and related to other potential morbidity, including lost time from work or school, pain, airway obstruction, and extension to deep neck spaces or the mediastinum, it is important to ensure that clinicians follow effective practices to decrease postoperative morbidity. This review assessed the evidence for the efficacy of systemic steroids to improve the patient experience and clinical outcomes for PTAs. We systematically reviewed the literature and conducted a meta-analysis to assess the evidence for the administration of steroids in PTAs.
Studies published in English prior to December 2014 were identified from MEDLINE, Scopus, and the Cochrane Register of Controlled Trials using search terms such as “peritonsillar abscess,” “peritonsillar infection,” “quinsy,” and “corticosteroids.” Only studies published in English were selected for review. The reference lists of identified studies were also checked to ensure that no relevant studies were missed.
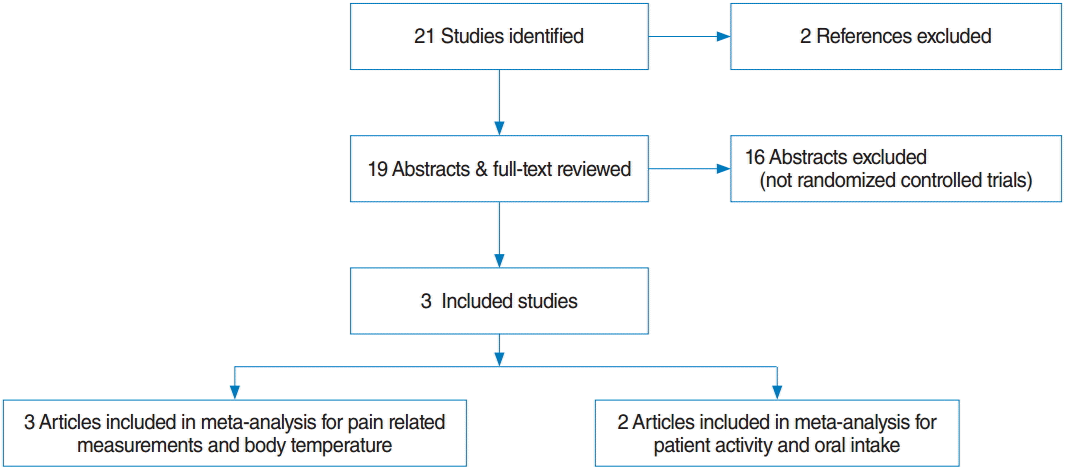
Two reviewers, working independently, screened all abstracts and titles for candidate studies and discarded studies that were not related to the administration of systemic steroids for treatment of PTAs. The full texts of potentially relevant studies were obtained if a decision for selection could not be made from the abstracts. Randomized controlled trials that met the following inclusion criteria were eligible for review: studies of patients suffering from PTAs who received any type of systemic steroid, such as dexamethasone and methylprednisolone, combined with antibiotics. Studies were not eligible if they included patients with bilateral PTAs or complications of PTAs such as airway compromise or involvement of another deep neck space, or if reports were duplicated. In addition, studies were excluded from the analysis if the outcomes of interest were not clearly reported with quantifiable data, or if it was not possible to extract and calculate the appropriate data from the published results. Fig. 1 summarizes the search strategy used to identify studies selected for meta-analysis.
Data from included studies were extracted using standardized forms and independently checked by 2 reviewers. The 2 pain-related primary outcomes were improvement in mean upper and lower incisor distance during mouth opening [9,10] and patientreported visual analog scale (VAS) pain score [11]. Outcomes for patients receiving a single, intravenous, high dose steroid bolus prior to antibiotic therapy were compared with those for patients in a control group (antibiotic only regimen). Secondary outcomes included body temperature (percentage or time to normalized temperature) [9-11], clinical outcomes with respect to patient activity (duration of hospitalization or time to normalized activity) [9,11], and degree of oral intake (time to swallow water or eat a normal diet without pain) [9-11] compared between 2 groups.
Mouth opening, recorded as the interincisor distance, has been reported to be an objective criterion for pain, and the VAS for pain has been well validated for measuring subjective pain levels in PTA management protocols. From the studies reporting the influence of systemic steroids on the clinical course of PTAs, we abstracted data regarding patient numbers, grading sc used, time to normalized temperature, percentage of improved patients (normalized mouth opening and body temperature, duration of hospitalization, resumption of normal diet or drinking without pain), and the P-value for the difference between the groups. Data reported only in graphical plots were not extracted for pooled meta-analysis unless specific numerical data were discernible or the authors of the relevant studies were able to verify the data. In the event of missing or incomplete data, attempts were made to obtain further details of the published results directly from the authors.
The risk of bias for each study was evaluated using the Cochrane ‘risk of bias’ tool, including selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), and reporting bias (selective reporting).
A meta-analysis of selected studies was performed with R ver. 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria). When original data were expressed as continuous variables (patient-reported pain score and degree of body temperature), meta-analysis was performed using the standardized mean difference (SMD) to calculate effect sizes. In all other cases, outcome incidence analysis was performed using the odds ratio (OR) calculated using the Mantel-Haenszel method. Heterogeneity was calculated with the I2 test. When significant heterogeneity among outcomes was found (defined as I2>50), the DerSimonian-Laird random-effects model was used. Those outcomes that did not present a significant level of heterogeneity (I2<50) were analyzed with the fixed-effects model. We used a funnel plot and Egger test simultaneously to detect potential publication bias. Additionally, we used Duval and Tweedie trim and fill method to adjust for missing studies and correct the overall effect size regarding publication bias.
A total of 3 studies with 153 participants were included and reviewed in this analysis. Overall patient characteristics could not be calculated owing to incomplete reporting of patient variables among the studies. The results of bias assessment and study characteristics are described in Table 1.
The percentage of improvement in trismus in patients at 4 hours (LogOR, 1.6842; P=0.0010), 12 hours (LogOR, 4.2187; P<0.0001), and 24 hours (LogOR, 4.4556; P<0.0001) during the posttreatment period (0–24 hours) was significantly higher in the steroid group than in the control group. Significant interstudy heterogeneity was not found (I2=0.00%). Although Egger test was not performed because of the small sample sizes in the selected studies, we suggest that the selected studies were not biased (Fig. 2A).
The patient-reported VAS pain scores at 24 hours (SMD, 0.8600; P=0.0095), 48 hours (SMD, 0.3900; P=0.2175), and 7 days (SMD, 0.3300; P=0.2967) during the posttreatment period (1–7 days) showed significant differences between the 2 groups exclusively at the 24-hour point. The I2 and Egger tests were not performed because of the small sample sizes in the selected studies (Fig. 2B).
The percentage of normalized body temperatures in patients at 12 hours (LogOR, 2.2344; P<0.0001), 24 hours (LogOR, 2.1223; P=0.0002), and 48 hours (LogOR, 2.7833; P<0.0001) during the posttreatment period (12–48 hours) was significantly higher in the steroid group than in the control group. Significant interstudy heterogeneity was not found (I2<50%). Although Egger test was not performed because of the small sample sizes in the selected studies, we suggest that the selected studies were not biased (Fig. 3A). By contrast, for the degree of body temperature measured during the posttreatment period (24–48 hours), only the measurement at the 24-hour point (SMD, 0.8700; P=0.0087) was significantly lower in the steroid group than in the control group except those at 48 hours (SMD, 0.0000; P=1.0000). The I2 and Egger tests were not performed because of the small sample sizes in the selected studies (Fig. 3B).
The clinical outcome with respect to duration of hospitalization (discharge rate) at 3 days (LogOR, 2.9996; P=0.0002), 4 days (LogOR, 3.0758; P<0.0001), and 5 days (LogOR, 2.9996; P=0.0002) during the posttreatment period (5 days) was significantly higher in the steroid group than in the control group (Fig. 4A). However, the percentage of patients reported as returned to normal activity at 24 hours (LogOR, 1.1200; P=0.0548), 48 hours (LogOR, 0.1900; P=0.737), and 7 days (LogOR, 0.0400; P=0.9436) during the posttreatment period (7 days) showed no significant difference between the steroid and control groups. The I2 and Egger’s tests were not performed because of the small sample sizes in the selected studies (Fig. 4B).
The percentage of patients swallowing water without pain at 4 hours (LogOR, 1.3775; P=0.0079), 12 hours (LogOR, 1.7275; P=0.0024), and 24 hours (LogOR, 2.1561; P<0.0001) during the posttreatment period (0–24 hours) was significantly higher in the steroid group than the control group. Significant interstudy heterogeneity was not found (I2=0.00%). Although Egger test was not performed because of the small sample sizes in the selected studies, we suggest that the selected studies were not biased (Fig. 5A).
The percentage of patients reported as returning to a normal diet at 24 hours (LogOR, 0.7455; P=0.1930), 48 hours (LogOR, 0.4305; P=0.4490), and 7 days (LogOR, 0.8177; P=0.1542) during the posttreatment period (7 days) was not significantly different between the steroid and control groups. The I2 and Egger tests were not conducted because of the small sample sizes in the selected studies (Fig. 5B).
Despite widespread use of antibiotics and surgical procedures for treating PTAs, severe pain, including odynophagia and trismus, remains a common and troubling clinical symptom [11,12]. Odynophagia is caused by inflammation of the superior constrictor muscle of the pharynx, which forms the lateral wall of the tonsillar fossa. Trismus is mainly due to inflammation and spasm of the medial pterygoid muscle. As a result of the spasm and pain, patients are unable to open their mouths and swallow saliva and drink; thus, dehydration can occur. Additionally, systemic signs such as fever may be present [10].
Corticosteroids are frequently used in a wide range of otolaryngologic conditions to overcome the inflammation process because of their strong antiinflammatory and antiedematous effects [13]. Corticosteroids also have a strong antipyretic effect. Steroids are regularly used to reduce upper aerodigestive tract edema resulting from trauma, surgery, infection, and anaphylaxis. Corticosteroid use as adjunctive therapy for other upper aerodigestive tract infections such as pharyngitis, epiglottitis, and tonsillitis is common. A recent meta-analysis reported that corticosteroids provided symptomatic relief of pain in sore throat, in addition to antibiotic therapy, mainly in participants with severe or exudative sore throat [6]. Although steroids have been used to treat edema and inflammation in other otolaryngologic diseases, their role in the treatment of PTAs has not been extensively studied.
In our study, focusing on the severe inflammatory and spasmodic components of the disease, we assessed body temperature, pain, duration of hospitalization (discharge rate or return to normal activity), and time to swallowing water or eating a normal diet without pain for a comparison between steroid and control groups. Pain assessment and relief is vital in the management of PTAs following local guidelines [2]. The VAS pain assessment scale, which allows the patient to mark a point along the scale that best represents their self-evaluated pain, is easy to use but subjective [11]. Therefore, the distance between the upper and lower incisor teeth during mouth opening (the degree of trismus) was adopted as an objective criterion for assessment of pain [9,10]. In this analysis, we used both subjective and objective approaches to assess the extent of pain.
Our results showed that trismus in patients during the posttreatment period (0–24 hours) was statistically improved in the steroid group compared with the control group, while patient-reported VAS pain scores during the posttreatment period (1–7 days) showed significant differences between the 2 groups exclusively at the 24-hour point. These findings show consistent results. However, the VAS pain scores at 24 hours (SMD, 0.8600; P=0.0095) tended to decrease compared with those at 48 hours (SMD, 0.3900; P=0.2175) and 7 days (SMD, 0.3300; P=0.2967) during the posttreatment period. These results imply that the effects of a single dose of corticosteroids on pain resoluresolution were most apparent in the initial 24 hours, which is similar to the results seen in sore throat and croup where a single dose is generally adequate [6].
In addition, the percentage of normalized body temperatures in patients during the posttreatment period (12–48 hours) was significantly higher in the steroid group than in the control group, and the degree of body temperature measured at the 24-hour point was significantly lower in the steroid group than in the control group. Although there was no significant difference in body temperature between the steroid and control groups at 48 hours post treatment, this finding could be explained by the fact that an adequate clinical response took place following 24 hours of broad-spectrum antibiotic therapy [5]. The results reported by Chau et al. [11] also showed that body temperature in both treatment and control groups normalized 24 hours after administration of antibiotics. In this study, the patients in the steroid group responded much more quickly to the treatment than the patients who received antibiotics alone, and their pain and fever subsided dramatically a few hours after steroid injection. As a result, there was a statistically significant difference between the groups with respect to duration of hospitalization and percentage patients able to swallow water without pain.
Subjective return to normal activity, although not a direct quality of life (QoL) measure, can reasonably be interpreted as a surrogate for overall QoL [11]. Chau et al. [11] found no significant difference between the steroid and control groups for this outcome. These findings contradict the results obtained for duration of hospitalization. Disparity between the outcomes used may explain this difference. However, the percentage of patients returning to normal activity at 24 hours after treatment (SMD, 1.1200; P=0.0548) tended to decrease compared with the percentage at 48 hours (SMD, 0.1900; P=0.7370) and 7 days (SMD, 0.0400; P=0.9436), though not significantly. Considering that an adequate clinical response took place following 24 hours of broad-spectrum antibiotic therapy, these results imply that the effects of a single dose of corticosteroids on improvement of QoL were most apparent in the initial 24 hours.
Although the results of our analysis detected statistically significant outcomes, there remains the dilemma that statistical improvement is not always clinically meaningful. The common representation of the effect size is Cohen d, which suggests that a larger effect size is more significant in clinically meaningful terms. An effect size between 2 means within a range closely encompassing 0.206 is considered small (possibly clinically nonsignificant), 0.506 is considered medium, and 0.806 or greater is considered large (and clinically significant) [14]. The effect sizes for measurements related to pain, fever, duration of hospitalization, and percentage of painless swallowing typically exceeded 0.8, and so were considered large effects. This finding appears consistent with the fact that there was a striking degree of synergy between antibiotics and steroids in the treatment of various head and neck infections, and these regimens were very effective for the treatment of PTAs.
Although the results of this study offer evidence for the use of systemic steroids for ameliorating symptoms and improving the clinical course of PTAs, it remains unclear if there were long-term benefits or adverse effects associated with use of corticosteroids, and a meta-analysis comparing these variables has yet to be conducted. In addition, had larger studies been undertaken, it may have been possible to reach more certain conclusions. Future research should focus on methodological quality and adequate powering. However, given the low morbidity, ease of administration, and relatively low cost of corticosteroid injection, it seems reasonable to conclude that systemic steroids along with antibiotic treatment may be applicable in all patients with PTAs.
In conclusion, steroids have been used traditionally to overcome the inflammatory process in certain otolaryngologic diseases, such as vocal fold edema, epiglottitis, Bell’s palsy, and sudden hearing loss, because of their strong anti-inflammatory and antiedematous effects. This study showed that a single intravenous dose of steroids, when used with antibiotics, might have positive effects on the clinical course of PTAs. However, our results were based on pooling trials with questionable methodological quality. Further trials with well-designed methodologies should be conducted to confirm our results.
REFERENCES
1. Herzon FS, Harris P. Mosher Award thesis. Peritonsillar abscess: incidence, current management practices, and a proposal for treatment guidelines. Laryngoscope. 1995; Aug. 105(8 Pt 3 Suppl 74):1–17.
2. Johnson RF, Stewart MG, Wright CC. An evidence-based review of the treatment of peritonsillar abscess. Otolaryngol Head Neck Surg. 2003; Mar. 128(3):332–43.

3. Steyer TE. Peritonsillar abscess: diagnosis and treatment. Am Fam Physician. 2002; Jan. 65(1):93–6.
4. Khayr W, Taepke J. Management of peritonsillar abscess: needle aspiration versus incision and drainage versus tonsillectomy. Am J Ther. 2005; Jul-Aug. 12(4):344–50.
5. Galioto NJ. Peritonsillar abscess. Am Fam Physician. 2008; Jan. 77(2):199–202.
6. Hayward G, Thompson M, Heneghan C, Perera R, Del Mar C, Glasziou P. Corticosteroids for pain relief in sore throat: systematic review and meta-analysis. BMJ. 2009; Aug. 339:b2976.

7. van Cauwenberge P, Van Hoecke H, Vandenbulcke L, Van Zele T, Bachert C. Glucocorticosteroids in allergic inflammation: clinical benefits in allergic rhinitis, rhinosinusitis, and otitis media. Immunol Allergy Clin North Am. 2005; Aug. 25(3):489–509.

8. Lamkin RH, Portt J. An outpatient medical treatment protocol for peritonsillar abscess. Ear Nose Throat J. 2006; Oct. 85(10):658. 660.

9. Ozbek C, Aygenc E, Tuna EU, Selcuk A, Ozdem C. Use of steroids in the treatment of peritonsillar abscess. J Laryngol Otol. 2004; Jun. 118(6):439–42.

10. Shaikh KR. Treatment of peritonsillar abscess and role of steriods. J Liaquat Univ Med Health Sci. 2008; Jan-Apr. 7(1):31–3.
11. Chau JK, Seikaly HR, Harris JR, Villa-Roel C, Brick C, Rowe BH. Corticosteroids in peritonsillar abscess treatment: a blinded placebo-controlled clinical trial. Laryngoscope. 2014; Jan. 124(1):97–103.

Fig. 2.
Systemic steroids versus control regarding pain. Odds ratio of the percentage of improvement of trismus at 4, 12, and 24 hours (A) from starting treatment, and standard mean difference in pain at 24 hours, 48 hours, and 7 days (B) from starting treatment. CI, confidence interval; Total, number of participants per group.
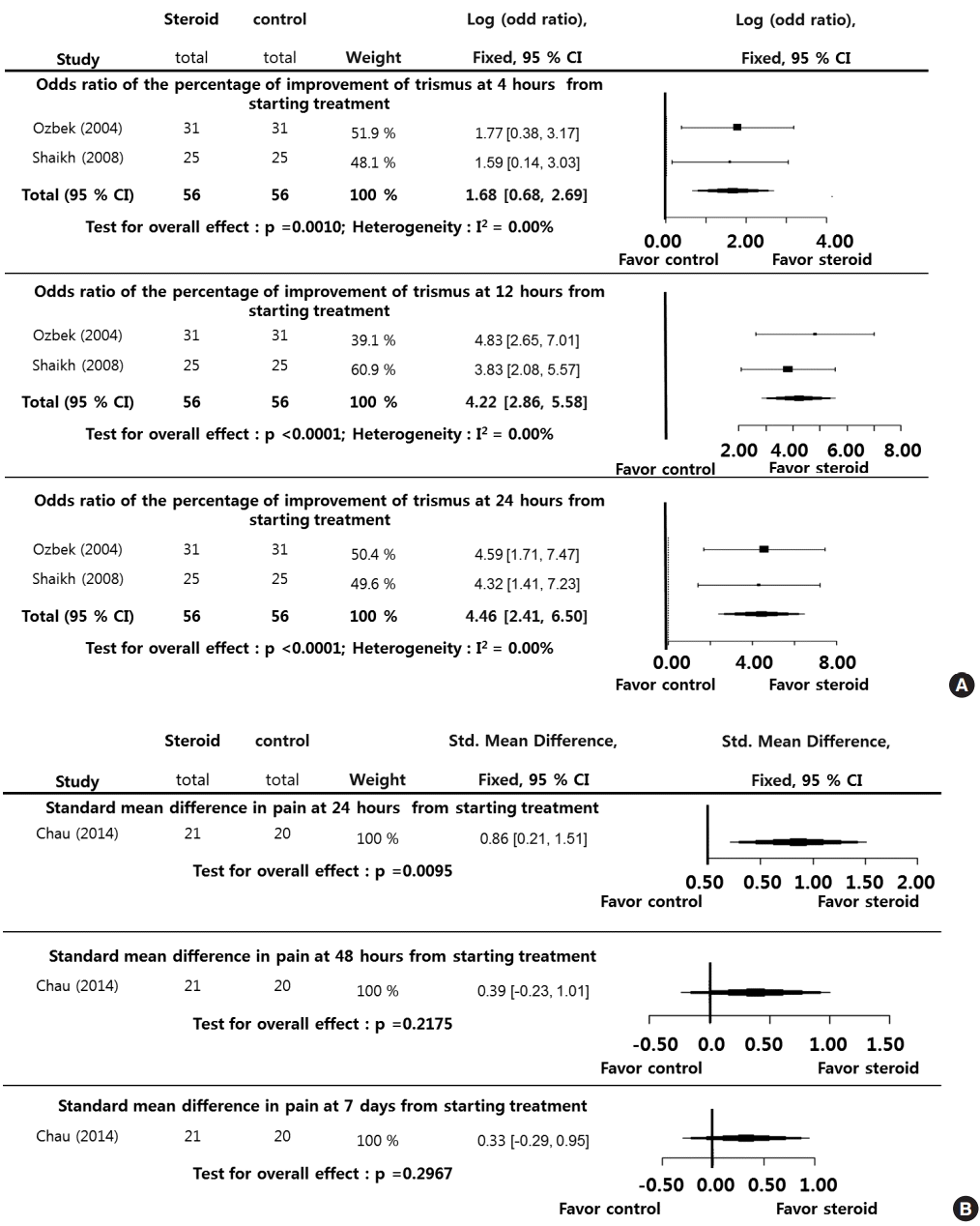
Fig. 3.
Systemic steroids versus control regarding body temperature. Odds ratio of the percentage of normalized body temperatures at 12, 24, and 48 hours (A) from starting treatment, and standard mean difference in the degree of body temperatures at 24 and 48 hours (B) from starting treatment. CI, confidence interval; Total, number of participants per group.
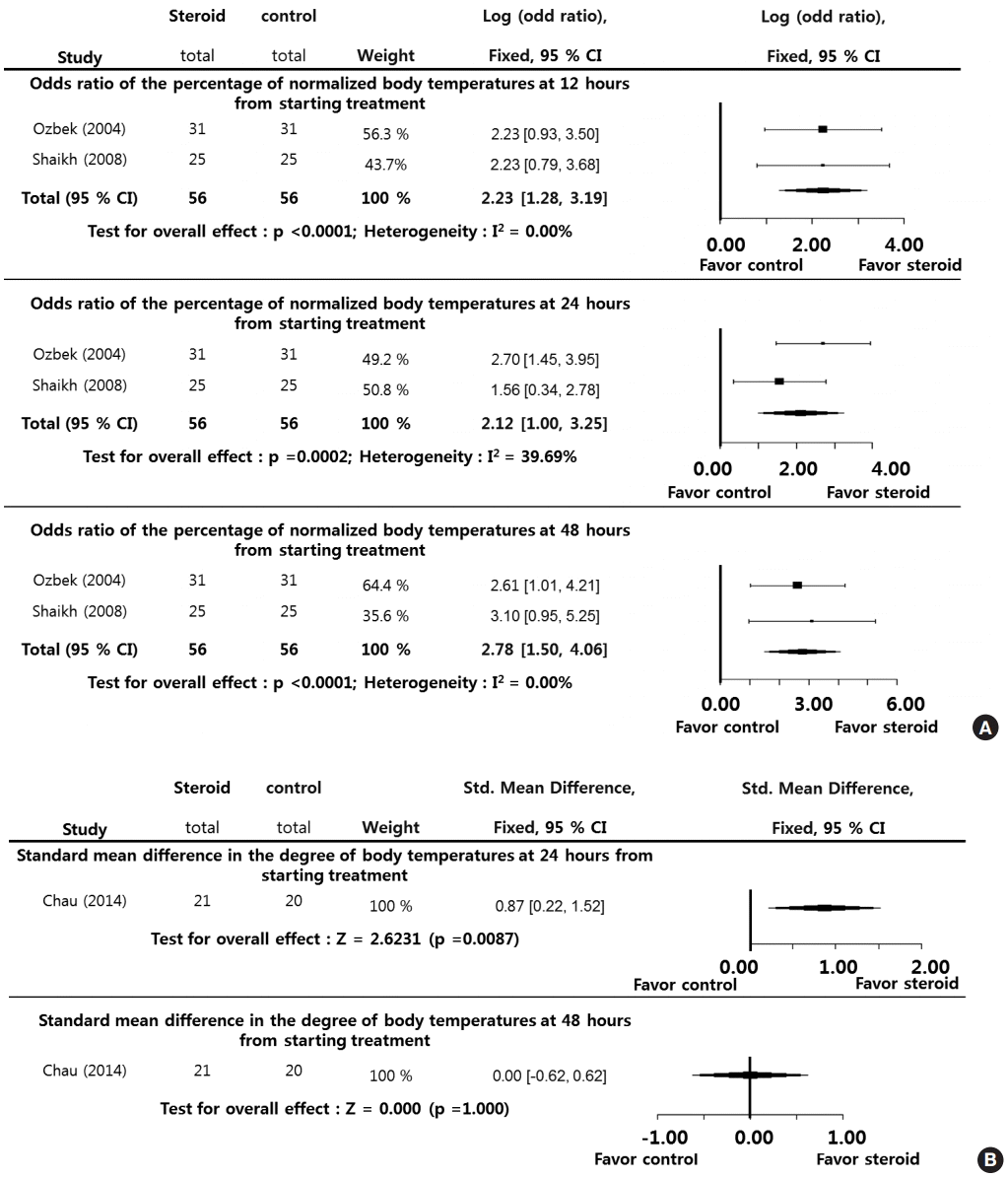
Fig. 4.
Systemic steroids versus control regarding patient recovery. Odds ratio of the discharge rate at 3, 4, and 5 days (A) from starting treatment, and odds ratio of the percentage of patients reporting return to normal activities at 24 hours, 48 hours, and 7 days (B) from starting treatment. CI, confidence interval; Total, number of participants per group.
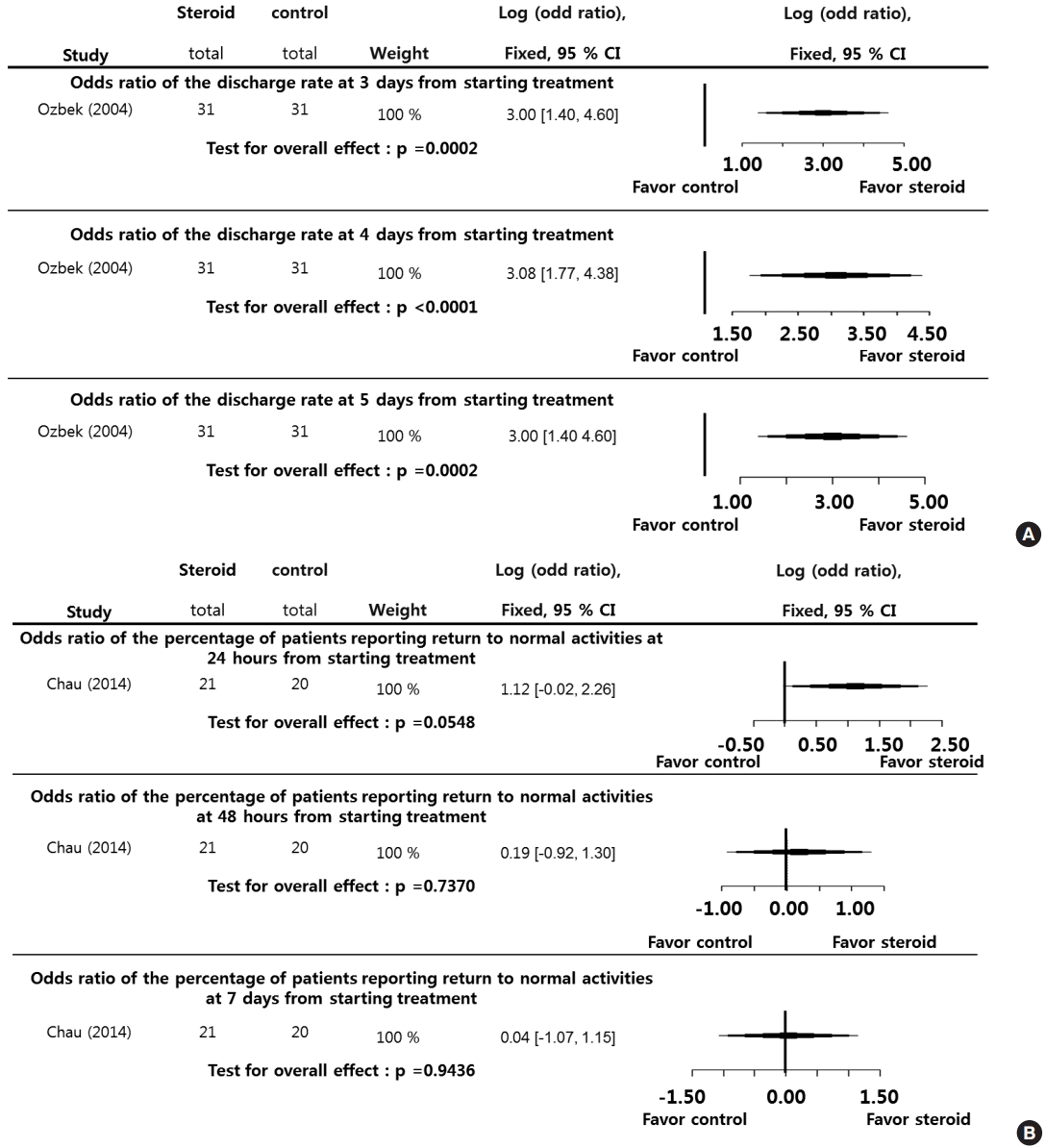
Fig. 5.
Systemic steroids versus control regarding dysphagia. Odds ratio of percentage of swallowing water without pain at 4, 12, and 24 hours (A) from starting treatment, and standard mean difference in the percentage of patients reporting return to a normal diet at 24 hours, 48 hours, and 7 days (B) from starting treatment. CI, confidence interval; Total, number of participants per group.
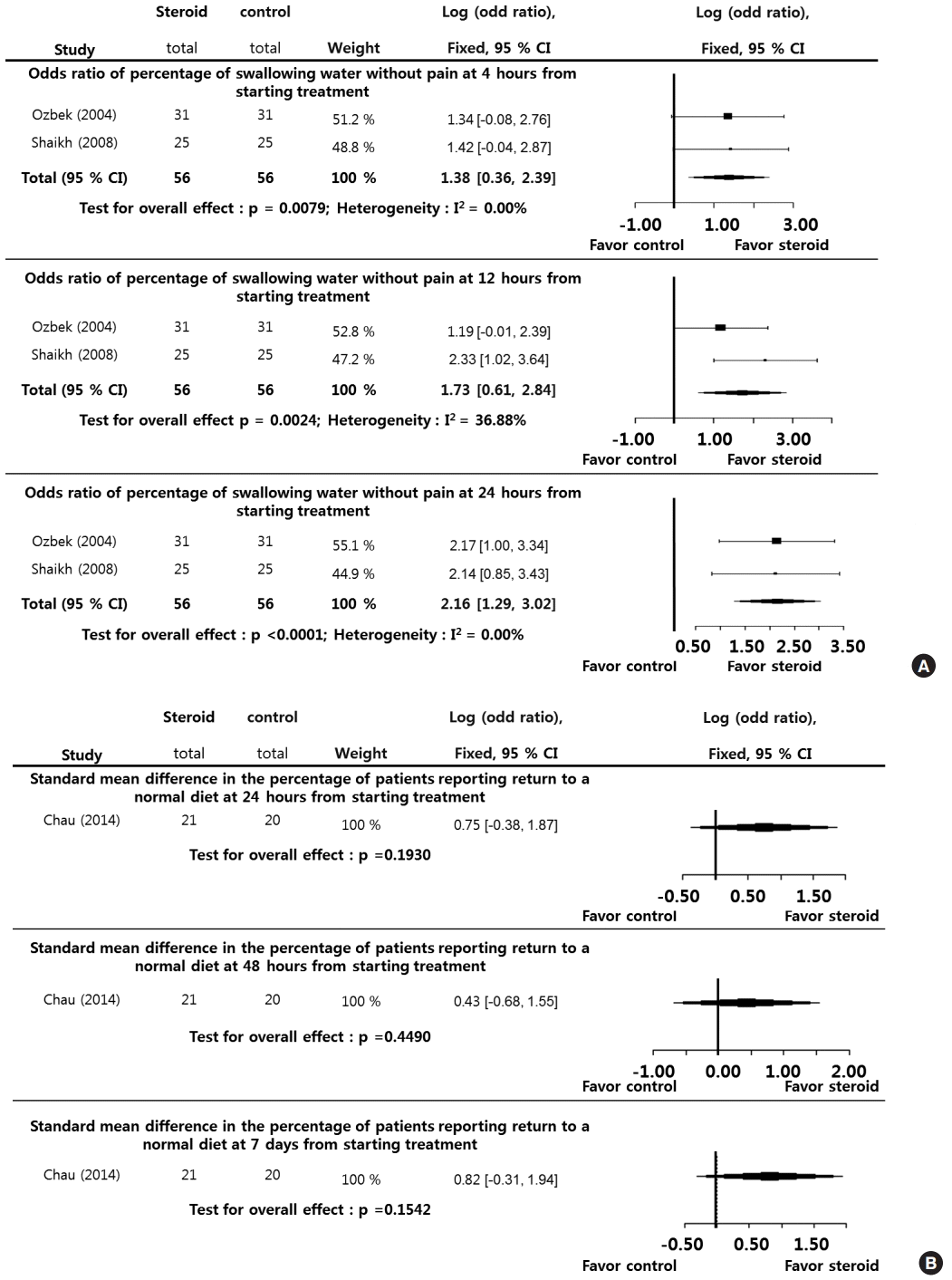
Table 1.
Summary of studies included in the meta-analysis
| Study | Sample size (n) | Comparison | Outcome measure analyzed | Judgment of risk of bias |
|---|---|---|---|---|
| Ozbek et al. [9] (2004) | 62 | Antibiotic plus steroid regimen vs. antibiotic only regimen | Pain-related measurement (percentage of improvement of trismus), percentage to normalized temperature, discharge rate, time to swallow water without pain | Unclear risk |
| Shaikh [10] (2008) | 50 | Antibiotic plus steroid regimen vs. antibiotic only regimen | Pain-related measurement (percentage of improvement of trismus), percentage to normalized temperature, time to swallow water without pain | Unclear risk |
| Chau et al. [11] (2014) | 41 | Antibiotic plus steroid regimen vs. antibiotic only regimen | Pain-related measurement (patient-reported pain score), time to normalized temperature, time to normalized activity, time to eat a normal diet without pain) | Low |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download