Abstract
Objectives
The purpose of study was to determine the clinical efficacy of primary tumor volume measurements of different primary sites in the oropharynx compared to the oral cavity.
Methods
A retrospective analysis of 85 patients with oral cavity or oropharynx cancer. The tumor area was manually outlined from axial magnetic resonance (MR) series. The software calculated the tumor volumes, automatically. The values of the primary tumor volumes were then subdivided into separate groups (≤3,500 mm3, >3,500 mm3).
Results
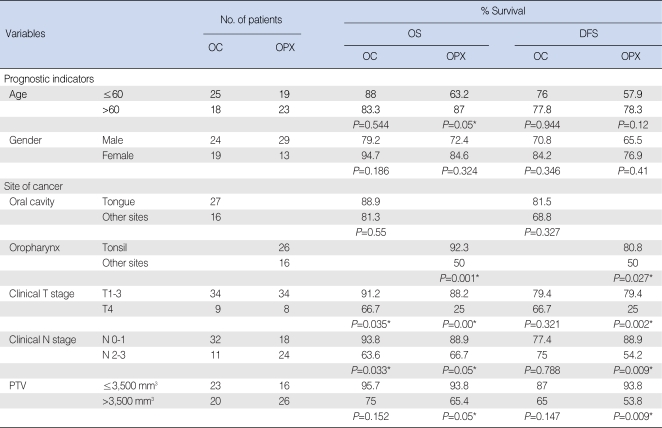
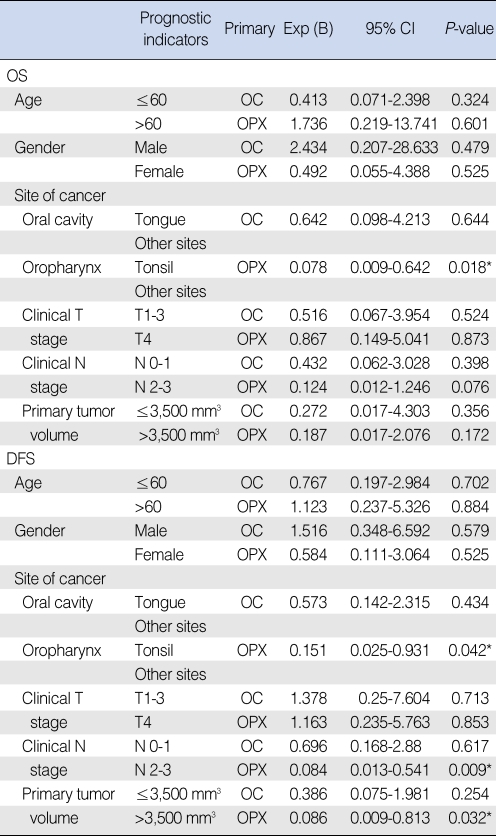
The prognostic indicators were the cT and cN (oral cavity); age, primary site, cT, cN, and primary tumor volume (oropharynx) on the univariate analysis. There was no significant prognostic factor for oral cavity cancer on the multivariate analysis. Primary site, cN, and primary tumor volume were independent prognostic indicators for oropharynx cancer by multivariate analysis.
The anatomic location of a primary malignancy significantly affects a patient's prognosis. The overall cure rate for patients with cancer of the oropharynx is usually low, and the use of surgery involves complex surgical procedures, including reconstruction. During the initial work up, patients with cancer of the oropharynx must be stratified to determine their prognosis and management. Currently, the TNM classification is the most commonly used system for stratifying the prognosis. However, the TNM system fails to define the true three-dimensional tumor volume within a given stage of disease.
Therefore, a superficial tumor with a good prognosis may be categorized in the same T-stage group as a deeply infiltrated tumor with a poor prognosis. Tumor volume is known to be a significant prognostic indicator for cancers of the head and neck region (1). However, there is controversy about using the tumor volume for guiding patient management.
The purpose of this study was to determine the clinical efficacy of the primary tumor volume, as measured by a 3D reconstruction program (Rapidia®, Infinite, Seoul, Korea), at different primary sites in the oropharynx, as compared with that of the primary tumor volume in the oral cavity.
We conducted a retrospective analysis of the patients with cancer of the oral cavity or oropharynx after they finished their primary treatment, and all the patients were treated at the Korea University Hospital from 1995 through 2007. The patients who had not finished their primary treatment, those who had a double primary cancer or who had non-epithelial cell types of cancer were excluded from this study. We also excluded the patients whose imaging scans could not be reconstructed by the 3D reconstruction program used for the study. Most of the excluded images were from old model computed tomography (CT)/magnetic resonance imaging (MRI) scanners that were not compatible with the 3D reconstruction program. Eighty-five patients were finally included in this study.
The study group was composed of 43 patients with oral cavity cancer (24 men and 19 women, mean age: 55.9 yr, range: 25 to 76 yr, mean follow up: 25.6 months, range: 1 to 79 months) and 42 patients with oropharynx cancer (29 men and 13 women, mean age: 59.6 yr, range: 28 to 85 yr, mean follow up: 33.3 months, range: 4 to 105 months). The primary sites of the oral cavity cancers were the tongue (n=27), the floor of the mouth (n=6), the buccal mucosa (n=3), the gingiva (n=2), the hard palate (n=1), the retromolar trigone (n=3) and the lip (n=1). The American Joint Committee on Cancer (AJCC) stage was Stage I in 8 patients, Stage II in 12, Stage III in 8 and Stage IV in 15 patients. The sites of the primary lesions in the patients with cancer of the oropharynx were the tonsil (n=26), the base of the tongue (n=13) and the soft palate (n=3). The AJCC stage was Stage I in 2 patients, Stage II in 6, Stage III in 8 and Stage IV in 26 patients.
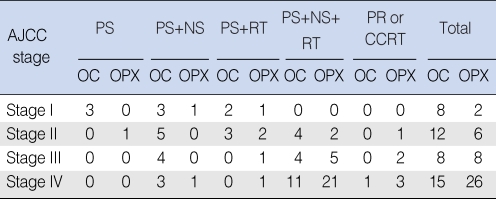
Our institutional therapy protocol differed based on the TNM stage, the surgical accessibility of the primary tumor, the response to radiation therapy, the postoperative pathology report and the patient's choice (Table 1). The patients with early stage disease underwent primary surgery or radiation alone. Elective neck treatment was performed if the risk of an occult neck metastasis was significant (Ipsilateral neck treatment if the tumor was lateralized and bilateral neck treatment if the tumor extended beyond the midline).
The patients with positive neck nodes received a therapeutic neck dissection. Postoperative radiotherapy was performed for the patients with close or involved resection margins, perineural or vascular invasion, multiple neck metastasis, an advanced stage or extracapsular nodal spread.
Combined modality treatments were provided for most of the advanced stage patients. However, some of the patients with clinical stage IV disease did not receive postoperative radiation therapy. One patient refused radiation therapy because of the concern about the potential complications. One patient was noted to have lung metastasis right after surgery. One patient with a history of a full course of radiation treatment at another head and neck site declined additional radiation therapy.
The tumor area was manually outlined from the best images of the pretreatment axial magnetic resonance (MR) series among the T1, T2 and gadolinium-enhanced T1-weighted images (Fig. 1). We used the CT images if there were no MRI scans available at the time of diagnosis (this was the case for 3 oral cavity cancer patients and 9 oropharynx cancer patients). A single board-certified otolaryngologist, who was kept "blind" to all the other clinical information, systematically outlined every primary lesion that was recognizable on each scan slice with the assistance of a head and neck radiologist who had 20 yr of experience. The software was used to automatically calculate the areas of the lesions and, from the thickness of the slice, the volume of tumor per slice and, finally, the sum of the volumes of all the slices. To reduce the measurement error, the same image was separately reviewed twice and the average value was regarded as the final tumor volume. The values of the primary tumor volumes were then subdivided into separate groups based on the ranked tumor volumes (≤3,500 mm3, >3,500 mm3) (Fig. 2).
The prognostic indicators we studied were age, gender, the site of the primary tumor, the primary tumor volume (≤3,500 mm3 or >3,500 mm3), the cT status and the cN status. All of the variables were first analyzed independently to estimate their effect on the overall survival (OS) (the interval between the date of diagnosis and the date of death from any cause or the most recent follow-up), and the disease-free survival (DFS) (from the end of treatment to the date of tumor recurrence or death from disease). The survival rates were calculated by the Kaplan-Meier method, and the differences between the survival curves were statistically analyzed with the log-rank test. The Cox model was used to determine the independent effects of the studied variables on survival. Associations between the primary tumor volume and neck metastasis (ipsilateral/contralateral) were examined by Fisher's exact test. A P-value of 0.05 or less was considered to be statistically significant.
Association of the primary tumor volume with the prognosis Fig. 2 shows the distribution of the primary tumor volume and this illustrates that there was substantial variation in the primary tumor volumes for lesions with the same T stage and there was a great deal of overlap for lesions with different T stages, and especially at the advanced stages. The results of the univariate and multivariate analyses for the prognostic factors for the OS and DFS are shown in Tables 2 and 3. When age, the primary site, the cT stage, the cN stage and the primary tumor volume were grouped as in Table 1, the prognostic indicators that were significantly associated with OS or DFS were the cT stage and cN stage (for oral cavity cancer); age, the site of the primary cancer, the cT stage, the cN stage and the primary tumor volume (for oropharynx cancer) were significant on the univariate analysis (Table 2). However, none of the factors were significant for the patients with oral cavity cancer on the multivariate analysis. However, the primary site, the cN stage and the primary tumor volume were independent prognostic factors for patients with oropharynx cancer on the multivariate analysis (Table 3). Fig. 3 demonstrates the difference in the survival plots between the patients with oral cavity cancers and the patients with the oropharynx cancers. The primary tumor volume, as a prognostic indicator, was significant only for the patients with oropharynx cancer. The patients with a large oropharynx tumor volume had a significantly poorer OS and DFS than those patients with a small oropharynx tumor volume.
The oropharyngeal primary tumor volume was significantly greater for patients with contralateral metastatic nodes as compared to the patients without metastasis (Fisher's exact test, P=0.004). However, there was no significant correlation with ipsilateral neck metastases (Fisher's exact test, P=0.443). In addition, there was no significant correlation between the primary tumor volume and the ipsilateral or contralateral lymph node metastasis in patients with oral cavity cancer (Fisher's exact test, P=0.255, P=0.161).
Our results show that the primary tumor volume was an independent prognostic indicator not only for survival, but also for local/regional disease recurrence. However, this correlation was observed for the patients with cancer of the oropharynx and not for the patients with oral cavity cancer. The findings of this study also demonstrated a correlation between the primary tumor volume and contralateral neck metastases in the patients with cancer of the oropharynx. Therefore, the primary tumor volume, as a prognostic indicator, is not applicable to all the head and neck regions. The differences in the tumor biology, the lymphatic distribution, the pattern of lymphangiogenesis and the mechanism of the lymphatic invasion may have contributed to the observed differences. Kimura et al. (1) have reported similar results; they showed a significant correlation between the tumor volume and lymph node metastasis for the patients with oropharyngeal or hypopharyngeal cancer, but not for the patients with oral or maxillary sinus cancer.
The primary tumor volume has often been studied as a prognostic indicator to complement the limitations of the two-dimensional AJCC staging system. Le Tourneau et al. (2) reported that the tumor volume should be considered as an essential prognostic indicator for patients with head and neck cancers. Mancuso et al. (3) reported that tumors ≥6 cm3 had a 52% local control rate as compared to an 89% control rate for those tumors <6 cm3 among 63 patients with supraglottic laryngeal cancer that was treated with definitive radiation therapy. Lo et al. (4) reported that the local control rate for >4 cm3 T2 laryngeal tumors was significantly lower than that for ≤4 cm3 T2 laryngeal tumors. Chao et al. (5) found that the tumor volume was the most important predictive factor for the IMRT response in patients with advanced oropharyngeal carcinoma. Doweck et al. (6) observed that the primary tumor volume was correlated with local disease control and survival for patients with head and neck cancer. Johnson et al. (7) studied 51 cases of advanced squamous cell carcinoma of the head and neck. They found an adverse effect of the tumor volume on the local/regional disease control. Johnson et al. (8) evaluated 76 patients with advanced head and neck cancer. They concluded that the tumor volume had better prognostic power than did the TNM stage. Chang et al. (9) reviewed 76 patients with T3/T4 nasopharyngeal cancer and they reported that the tumor volume was the only independent prognostic variable identified.
However, measuring the tumor volume is a laborious process that is performed by manual tracing the tumor outline. The original technique for evaluating the tumor volume was complex and time-consuming. Moreover, tumor volume measurement is rather subjective and inter-reader or intrareader errors are possible (1, 10). However, Gordon et al. (11) showed that these errors were not significant when performing MR imaging-based tumor volume measurement. In addition, the recent advances in radiology imaging and the three-dimensional treatment-planning systems have enabled us to quantify the tumor volume more easily and accurately. We used a 3D reconstruction program to measure the tumor volume, and it was a fast, reproducible and reliable method.
Most of the primary tumor volume measurements were calculated with using the MR images. If a MR image was not available, then we used a CT image. The MR images have been shown to be more accurate for measuring tumor volume than using the CT images, and especially for tumors of the oral cavity and oropharynx, if the tissue contrast between the tumor and the surrounding tissue is insufficient on the CT images (12-15% of the cases) or if artifacts cover the tumor on the CT images or if the tumor is very small (stage T1 or T2) (12). However, some reports have shown that the tumor volume of pharyngolarynx tumors, as calculated with the CT and MR imaging, did not exhibit significant differences (13).
The limitations of this study include that the tumor volume analysis was not stratified separately based on the different T stages, the anatomic subsites and the various treatment modalities due to the small number of patients. Other limitations were the relatively short follow-up period and the study's retrospective design. Therefore, prospective analysis of a large cohort of patients is warranted.
In summary, the results of this study demonstrate that the primary tumor volume measurement was a reliable method for stratifying the outcomes of patients with oropharynx tumor. The absolute measures of the tumor volume by the 3D reconstruction program were significantly predictive of the OS and DFS for patients with oropharynx cancer, but not for the patients with oral cavity cancer. The addition of the primary tumor volume measurement to the current AJCC staging system can compensate for some of the limitations of AJCC staging. However, its usefulness depends on the primary site of the tumor in the head and neck region.
References
1. Kimura Y, Sumi M, Ichikawa Y, Kawai Y, Nakamura T. Volumetric MR imaging of oral, maxillary sinus, oropharyngeal, and hypopharyngeal cancers: correlation between tumor volume and lymph node metastasis. AJNR Am J Neuroradiol. 2005; 10. 26(9):2384–2389. PMID: 16219850.
2. Le Tourneau C, Velten M, Jung GM, Bronner G, Flesch H, Borel C. Prognostic indicators for survival in head and neck squamous cell carcinomas: analysis of a series of 621 cases. Head Neck. 2005; 9. 27(9):801–808. PMID: 16086415.

3. Mancuso AA, Mukherji SK, Schmalfuss I, Mendenhall W, Parsons J, Pameijer F, et al. Preradiotherapy computed tomography as a predictor of local control in supraglottic carcinoma. J Clin Oncol. 1999; 2. 17(2):631–637. PMID: 10080608.

4. Lo SM, Venkatesan V, Matthews TW, Rogers J. Tumour volume: implications in T2/T3 glottic/supraglottic squamous cell carcinoma. J Otolaryngol. 1998; 10. 27(5):247–251. PMID: 9800621.
5. Chao KS, Ozyigit G, Blanco AI, Thorstad WL, Deasy JO, Haughey BH, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: impact of tumor volume. Int J Radiat Oncol Biol Phys. 2004; 5. 01. 59(1):43–50. PMID: 15093897.

6. Doweck I, Denys D, Robbins KT. Tumor volume predicts outcome for advanced head and neck cancer treated with targeted chemoradiotherapy. Laryngoscope. 2002; 10. 112(10):1742–1749. PMID: 12368607.

7. Johnson CR, Thames HD, Huang DT, Schmidt-Ullrich RK. The tumor volume and clonogen number relationship: tumor control predictions based upon tumor volume estimates derived from computed tomography. Int J Radiat Oncol Biol Phys. 1995; 9. 30. 33(2):281–287. PMID: 7673015.
8. Johnson CR, Khandelwal SR, Schmidt-Ullrich RK, Ravalese J 3rd, Wazer DE. The influence of quantitative tumor volume measurements on local control in advanced head and neck cancer using concomitant boost accelerated superfractionated irradiation. Int J Radiat Oncol Biol Phys. 1995; 6. 15. 32(3):635–641. PMID: 7790249.

9. Chang CC, Chen MK, Liu MT, Wu HK. The effect of primary tumor volumes in advanced T-staged nasopharyngeal tumors. Head Neck. 2002; 10. 24(10):940–946. PMID: 12369073.

10. Weiss E, Hess CF. The impact of gross tumor volume (GTV) and clinical target volume (CTV) definition on the total accuracy in radiotherapy theoretical aspects and practical experiences. Strahlenther Onkol. 2003; 1. 179(1):21–30. PMID: 12540981.
11. Gordon AR, Loevner LA, Shukla-Dave A, Redfern RO, Sonners AI, Kilger AM, et al. Intraobserver variability in the MR determination of tumor volume in squamous cell carcinoma of the pharynx. AJNR Am J Neuroradiol. 2004; Jun–Jul. 25(6):1092–1098. PMID: 15205156.
12. Lenz M, Greess H, Baum U, Dobritz M, Kersting-Sommerhoff B. Oropharynx, oral cavity, floor of the mouth: CT and MRI. Eur J Radiol. 2000; 3. 33(3):203–215. PMID: 10699737.

13. Daisne JF, Duprez T, Weynand B, Lonneux M, Hamoir M, Reychler H, et al. Tumor volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology. 2004; 10. 233(1):93–100. PMID: 15317953.

Fig. 1
An example of measuring the primary tumor volume with using the 3D reconstruction program. (A) The gadolinium-enhanced, T1-weighted axial MR images of a tonsil cancer patient and (B) manual tracing of the area of the tumor. The tumor area is manually outlined (blue line) from the sequential sections. This allows the software to automatically calculate the areas of the lesions and, from the thickness of the slice, the volume of the tumor per slice and finally, the sum of the volumes of all the slices. (C) Pre-tracing the CT image of the same patient and (D) post-tracing the CT image.
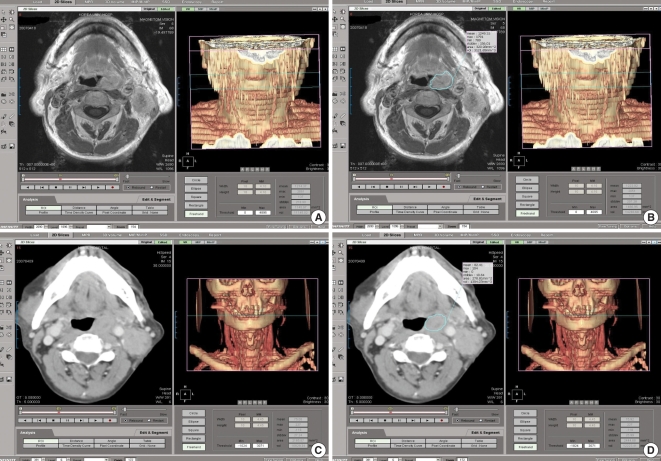
Fig. 2
Distribution of the primary tumor volumes in the oral cavity (A) and the oropharynx (B). There was substantial variation of the primary tumor volume for the same stage lesions and a great deal of overlap among tumors at different stages, and especially those at the advanced stage. Therefore, the two-dimensional T stages do not reflect the actual three-dimensional tumor volume. We subdivided the patients into two volume groups based on the cutoff value of 3,500 mm3.
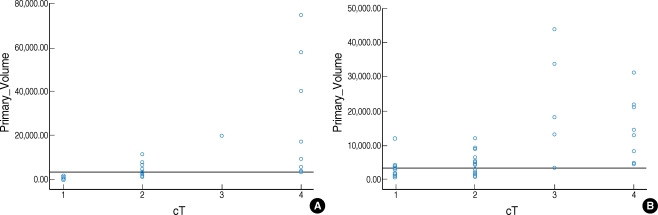
Fig. 3
Comparison of the survival plots between the patients with oropharynx and those with oral cavity cancers. (A) The Kaplan-Meier plot of the disease-free survival as assessed by the primary tumor volume and according to univariate analysis, P=0.009 (oropharynx cancer), (B) the overall survival as assessed by the primary tumor volume and according to univariate analysis, P=0.05 (oropharynx cancer), (C) multivariate study with Cox proportional hazard multivariate analysis, P=0.032 (oropharynx cancer) and (D) the Kaplan-Meier plot of the disease-free survival according to the primary tumor volume. As compared to the patients with oropharynx cancer, there was no significant relationship between the primary tumor volume and disease recurrence for the patients with oral cavity cancers (univariate test, P=0.147, oral cavity cancer).
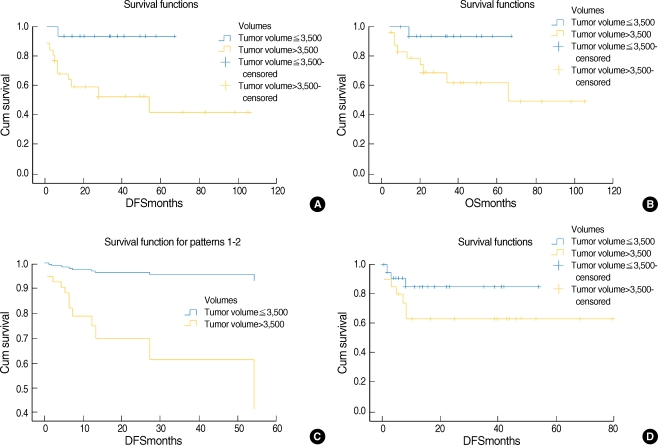
Table 1
The treatment modality that was performed at various stages of the oral cavity/oropharynx cancer. The patients with early stage disease underwent primary surgery or radiation alone. Elective neck treatment was done if the risk of an occult neck metastasis was significant (Ipsilateral neck treatment if the tumor was lateralized and bilateral neck treatment if the tumor extended beyond the midline). The patients with a clinically positive neck node received therapeutic neck dissection. The patients with lymph node metastasis, resection margins with tumor invasion or an advanced tumor stage underwent postoperative radiotherapy




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download