This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
Aminoglycosides are commonly used antibiotic agents, and they are known to generate free oxygen radicals within the inner ear and to cause vestibulo-cochlear toxicity and permanent damage to the sensory hair cells and neurons. Melatonin, a pineal secretory product, has the properties of being a powerful direct and indirect antioxidant. The aim of the present study was to prove the antioxidant effect of melatonin against gentamicin-induced ototoxicty.
Methods
The utricular maculae of Sprague-Dawley rats were prepared from postnatal day 2-4, and these maculae were were divided into 6 groups as follows: 1) control, 2) melatonin only, 3) gentamicin only, and 4), 5), and 6) gentamicin plus melatonin (10, 50, and 100 µM, respectively). To count the number of hair cells, 5 utricles from each group were stained with phalloidin-FITC on the 1st, 4th, and 7th days after drug administration. Reactive oxygen species (ROS) was assessed by using the fluorescent probe hydrofluorescent diacetate acetyl ester. The caspase-3 activity was also examined with using the fluorescent caspase-3 substrate and performing Western blotting.
Results
The result of this study showed that gentamicin induced the loss of utricular hair cells, and this loss of hair cells was significantly attenuated by co-administration of melatonin. Melatonin reduced ROS production and caspase-3 activation in the gentamicin treated utricular hair cells.
Conclusion
Our findings conclusively reveal that melatonin has protective effects against gentamicin-induced hair cell loss in the utricles of rat by inhibiting both ROS production and caspase-3 activity.
Go to :

Keywords: Melatonin, Ototoxicity, Antioxidants, Utricle
INTRODUCTION
Aminoglycoside antibiotics, including gentamicin, have various ototoxic effects on the inner ear organ, and these effects cause hearing loss and vestibulopathy (
1). Apoptosis is known as the predominant mode of hair cell loss that caused by these aminoglycoside antibiotics and their ototoxicity, and reactive oxygen species (ROS) play an important role as a promoter of apoptosis (
2,
3). ROS include oxygen ions, free radicals and peroxides that are both inorganic and organic. They have been implicated in apoptosis, DNA damage, suppression of an antioxidant expression and induction of programmed cell death (
4).
On the other hand, melatonin, which is a secretary product of the pineal gland, was recently found to be an antioxidant and a free radical scavenger (
5). It is a highly effective direct scavenger of reactive radicals and their intermediates such as hydrogen peroxide, singlet oxygen species, nitric oxide, peroxynitrite anions and peroxynitrous acid. Not only does melatonin act as the direct scavenger, but it also participates as an indirect antioxidant. It induces superoxide dismutase, glutathione peroxidase, glutathione reductase and glucose-6-phosphate dehydrogenase, and it inhibits γ-glutamylcysteine synthase and nitric oxide synthase (
4,
6). Owing to its lipophilic characteristic that allows it to cross the blood-brain barrier, its highly efficient free radical scavenging properties and its capacity for inducing antioxidative enzymes, melatonin has been proposed to be an endogenous protective agent against oxidative damage to the brain (
7).
Accordingly, we could hypothesize that melatonin has an anti-apoptotic effect on the gentamicin-induced hair cell loss in rat utricle, and this is mainly caused via reducing oxidative stress and the activity of caspase.
Go to :

MATERIALS AND METHODS
Animals and tissue culture
The utricles from Sprague-Dawley rats were obtained at the postnatal 2nd-4th day. After removing the temporal bones from the rat, the utricular maculae were identified and separated, and the otoconial membranes were gently removed. The dissection procedure was performed in Dulbecco's Phosphate Buffered Saline (DPBS) under a stereomicroscope (OLYMPUS LG-PS2, Tokyo, Japan) at room temperature. The explants were placed with their epithelial surfaces upper most and flattened and the explants were anchored with a gelatin layer coating to allow them to adhere to the dish. The explants were incubated at 37℃ in a humidified 5% carbon dioxide atmosphere. The culture media was changed every second day.
Experimental groups
The explants were divided into 6 groups as follows.
C group: the explants were cultured in the Dulbecco's modified eagle's medium (DMEM) culture media.
G group: gentamicin (Sigma, St. Louis, MO, USA) 1 mM was added to the culture media during the first two culture days.
M group: melatonin (Sigma, St. Louis, MO, USA) 50 µM was added to the culture media for the entire culture period.
GM1 group: gentamicin (Sigma) 1 mM was added to the culture media during the first two culture days and melatonin (Sigma) 10 µM was added to the culture media for the entire culture period.
GM2 group: gentamicin (Sigma) 1 mM was added to the culture media during the first two culture days and melatonin (Sigma) 50 µM was added to the culture media for the entire culture period.
GM3 group: gentamicin (Sigma) 1 mM was added to the culture media during the first two culture days and melatonin (Sigma) 100 µM was added to the culture media for the entire culture period.
Counting the hair cells was performed in the utricles of each group at the 1st, 4th, and 7th days. The sites used for counting the utricular hair cells were those sites where the most cells were observed under the microscope. All the statistical analysis was performed using the Mann-Whitney test. For analysis, the criterion for statistical significance was set at P-value ≤0.05.
Phalloidin-FITC staining
The explants were fixed with 4% paraformaldehyde at 4℃ for 1 hr and then they were washed with 0.1 M DPBS (pH 7.4). They were treated with 0.25% Triton X-100 (Sigma, St. Louis, MO, USA) at room temperature for 3 min and then they were washed with 0.1 M PBS (pH 7.4). They were stained with 1.0 µg/mL phalloidin-fluorescein isothiocyanate (phalloidin-FITC, Sigma) for 40 min in a dark room and then they were washed three times with 0.1 M PBS (pH 7.4). The slides were covered with aluminum foil. The utricular explants were observed using a confocal laser scanning microscope (CLSM, ZEISS510 META, Gottingen, Germany) with appropriate filters for FITC (absorption 494 nm, emission: 518 nm).
Measurement of ROS
The ROS activity was determined by using hydrofluorescent diacetate acetyl ester (H2DCFDA, Molecular probes, Eugene, OR, USA) at 37℃ in the C, G, GM2, and M groups. The utricular explants were stained with 10 µM H2DCFDA for 30 min in a tissue incubator (at 37℃ in a humidified 5% carbon dioxide atmosphere). The utricular explants were observed using a CLSM with appropriate filters for H2DCFDA (absorption 488 nm, emission 530 nm). The green fluorescence appears on the utricular hair cells that express ROS. The measurement of ROS was performed at the 3rd day after beginning the utricle culture.
Caspase-3 activity measurement
The caspase-3 activity was assayed using a CaspGLOW fluorescein active caspase-3 staining kit (BioVision, Mountain View, CA, USA). The caspase-3 activity measurements were performed for the explants from groups C, G, GM2, and M. Double-staining was done using phalloidin-FITC (green) and fluorescent caspase substrate (red). The stained utricular explants were observed using a CLSM with appropriate filters for FITC and the caspase substrate (absorption 540 nm, emission 570 nm). Measuring the caspase-3 activity was performed at the 3rd day after beginning the utricle culture.
Western blot analysis
The utricles obtained from 50 rats were divided into groups C, G, M, and GM2, and these were cultured for 24 hr. The utricles were washed with PBS and then homogenized in 1 mL Radio-Immuno Precipitation (RIPA) buffer (150 mM NaCl, 1% NP-40, 50 mM Tris, pH 8.0, 1 mM EGTA, 0.5% Deoxycholate) with 100 µg/mL phenylmethylsulfonyl fluoride and 1 µg/mL leupeptin to estimate the caspase-3 activation and the cell suspensions, cultures were clarified by centrifugation at 15,000 rpm for 30 min. The pellets were re-extracted and the supernatants were pooled. The amount of protein was estimated by a Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). Tissues lysates that contained 20 µg of total protein were load onto 12% sodium dodesyl sulfate (SDS)-polyacrylamide gel and then the proteins were transferred to polyvinylidine difluoride (PVDF) membranes (Amersham, Arlington Heights, IL, USA). Each membrane was blocked with blocking buffer (5% skim milk in TTBS: 137 mM NaCl, 0.1% Tween 20 and 20 mM Tris-HCl, pH 7.6) for 1 hr at room temperature and the membranes were incubated with the primary antibody (anti-caspase-3 1:1,000, anti-GAPDH 1:1,000, Santacruz, CA, USA), and then the membranes were incubated at room temperature for 1 hr with a secondary antibody conjugated with HRP (1:10,000, diluted in 5% skim milk). The signal was detected using an ECL Western blotting detection system (GE Healthcare, Amersham, UK). Western blot analysis was performed at the 3rd day after beginning the utricle culture.
Go to :

RESULTS
The number of utricular hair cells
The numbers of live hair cells stained with phalloidin-FITC and seen with the CLSM were 292.8±21.8 (n=5), 292.0±10.1 (n=5) and 288.4±8.0 (n=5), respectively, in group C at the 1st, 4th, and 7th days (
Fig. 1).
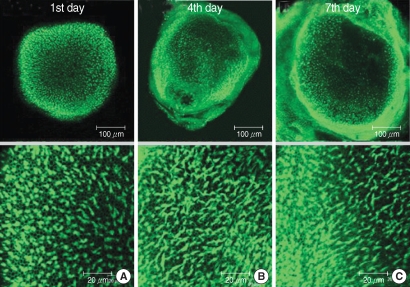 | Fig. 1Photomicrographs showing utricular organotypic cultures labeled phalloidin-FITC in control group. Left column shows rat's utricle at the 1st day after utricle culture (A). Median (B) and right (C) column are the 4th and 7th days'utricle findings. The number of live hair cells were 292.8±21.8 (n=5), 292.0±10.1 (n=5), and 288.4±8.0 (n=5) in group C at the 1st, 4th, and 7th day after culture stated. Scale bar shown in each panels. 
|
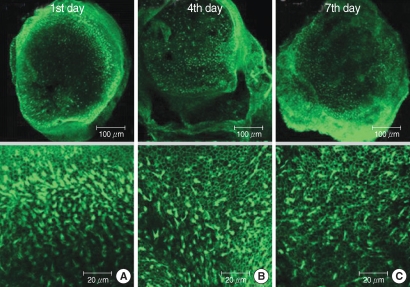
The numbers of live hair cells in group G were 48.2±15.9 (n=5), 38.4±8.1 (n=5), and 19.8±11.1 (n=5), respectively, at the 1st, 4th, and 7th days (
Fig. 2).
 | Fig. 2Photomicrographs showing utricular organotypic cultures labeled phalloidin-FITC in gentamicin (G) group. The number of live hair cells in group G were 48.2±15.9 (n=5), 38.4±8.1 (n=5), and 19.8±11.1 (n=5) at 1st, 4th, and 7th day after culture stated. The utricles cultured for 48 hr in the presence of 1 mM gentamicin show extensive loss of hair cells. 
|
Group G demonstrated a smaller number of live hair cells as compared to that of group C (
P<0.01,
Fig. 3).
 | Fig. 3Mean number of utricular hair cells per 20,000 µm2 in each the groups. Melatonin-treated group was not significantly different from control group. GM2 & GM3 groups had more hair cells than G group significantly. 
|
The numbers of live hair cells in group M were 281.0±11.2 (n=5), 276.0±22.2 (n=5), and 277.6±15.2 (n=5), respectively at the 1st, 4th, and 7th days after culture stated and the average was 278.2±15.7 (n=15) (
Fig. 4). There was no significant difference for the live cell numbers between groups M and C (
P>0.05,
Fig. 3).
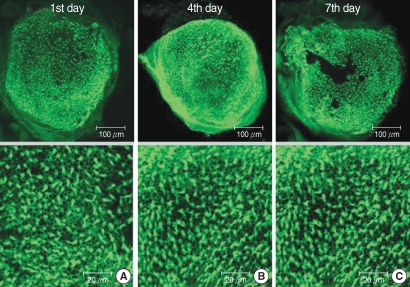 | Fig. 4Photomicrographs showing utricular organotypic cultures labeled phalloidin-FITC in melatonin (M) group. The number of live hair cells in group M were 281.0±11.2 (n=5), 276.0±22.2 (n=5), and 277.6±15.2 (n=5) at the 1st, 4th, and 7th day after culture stated. Utiricular hair cells were observed as many as control group. 
|
The numbers of live hair cells in group GM1 that was stained by phalloidin-FITC and seen by the CLSM were 58.2±10.1 (n=5), 54.2±5.5 (n=5), and 33.0±6.4 (n=5), respectively, at the 1st, 4th, and 7th days after culture stated, and the average was 48.5±13.4 (n=15) (
Fig. 5). The numbers of live hair cells in group GM2 that were stained phalloidin-FITC and seen by the CLSM were 130.6±13.4 (n=5), 118.4±13.4 (n=5), and 103.2±19.0 (n=5), respectively, at the 1st, 4th, and 7th days after culture stated, and the average was 117.4±20.9 (n=15) (
Fig. 6). The numbers of live hair cells in group GM3 that were stained with phalloidin-FITC and seen by the CLSM were 168.6±19.5 (n=5), 161.0±22.0 (n=5), and 154.4±14.3 (n=5), respectively, at the 1st, 4th, and 7th days, and the average was 161.3±15.7 (n=15) (
Fig. 7).
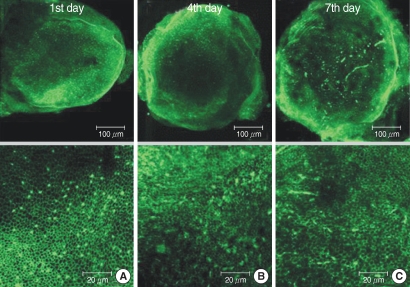 | Fig. 5Photomicrographs showing utricular organotypic cultures labeled phalloidin-FITC in GM1 group. The number of live hair cells in group GM1 were 58.2±10.1 (n=5), 54.2±5.5 (n=5), and 33.0±6.4 (n=5) at the 1st, 4th, and 7th day after culture stated. The utricles show extensive loss of hair cells. 
|
 | Fig. 6Photomicrographs showing utricular organotypic cultures labeled phalloidin-FITC in GM2 group. The number of live hair cells in group GM2 stained phalloidin-FITC by CLSM were 130.6±13.4 (n=5), 118.4±13.4 (n=5), and 103.2±19.0 (n=5) at the 1st, 4th, and 7th day after culture stated. The utricular hair cells were observed more than GM1 group. 
|
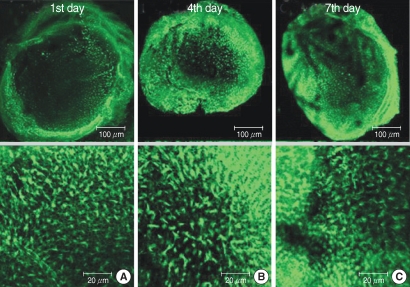 | Fig. 7Photomicrographs showing utricular organotypic cultures labeled phalloidin-FITC in GM3 group. The number of live hair cells in groups GM3 were 168.6±19.5 (n=5), 161.0±22.0 (n=5), and 154.4±14.3 (n=5) at the 1st, 4th, and 7th day after culture stated. The utricular hair cells were observed more than GM1 & GM2 group, significantly. 
|
The numbers of live hair cells of groups GM1-3 were significantly smaller than that of group C (
P<0.01,
Fig. 3). The numbers of live hair cells in groups GM2 and GM3 were significantly more than those of group G (
P<0.05), whereas group GM1 didn't show a significant difference for the number of live hair cells (
P>0.05,
Fig. 3).
Effects of melatonin on ROS generation in the utricles
To determine the extent of ROS generation in the utricles, the fluorescence activity of H2DCFDA, which is a marker of ROS, was evaluated in four groups: groups C, G, M, and GM2 (
Fig. 8). A weak ROS expression was observed in groups C and M, whereas a strong expression was seen in group G. Group G presented the greatest ROS expression. The fluorescent signal in group GM2 (1 mM gentamicin pretreatment together with 50 µM melatonin treatment) was less intense than that of group G (without melatonin treatment).
 | Fig. 8Effects of melatonin on gentamicin-induced production of reactive oxygen species (ROS). Photomicrographs showing ROS activation in control (A), 1 mM gentamicin only (B), 50 µM melatonin only (C), and 1 mM gentamicin plus 50 µM melatonin (D). Scale bar shown in panels. 
|
Increased caspase-3 activity in the gentamicin treated group
Group G exhibited a strong signal for fluorescent activated caspase-3. Group GM2. which was exposed to 1 mM gentamicin and 50 µM melatonin, showed less intense staining for activated caspase-3, as compared with group G. Weak caspase-3 activation also appeared around the utricular hair cells in groups C and M (
Fig. 9).
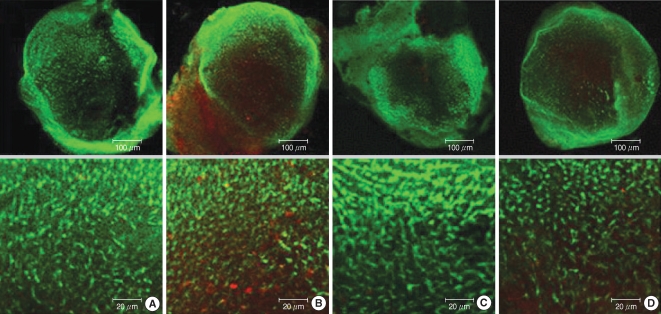 | Fig. 9Photomicrographs showing red caspase-3 substrate activation in normal utricular culture (A column), utricular cultures treated with 1 mM gentamicin (B column), utricular cultures treated with 50 µM melatonin (C column), and utricular cutures treated with gentamicin 1 mM plus 50 µM melatonin (D column). Caspase-3 expression of utricle presented in following order of intensity, group G (B), group GM2 (D), group M (C), and group C (A). Scale bar shown in panels. 
|
Western blot analysis following RIPA extraction demonstrated the presence of caspase-3 as 32 kDa bands in the groups. Group C weakly expressed a caspase-3 band. Group G, which is treated with gentamicin, showed more intense caspase-3 activity, as compared with groups GM and M. For the western blotting results, the caspase-3 expression was shown in order of intensity as groups C, M, GM, and G (
Fig. 10).
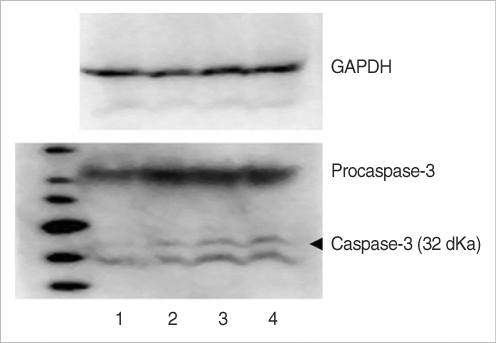 | Fig. 10The protein of caspase-3 on western blotting were detected in rat vestibular hair cells. 1: control, 2: group M, 3: group GM2, 4: group G. Group C was weakly expressed caspase-3 band. Group G was increased caspase-3 activation compared to group GM and M. 
|
Go to :

DISCUSSION
It is well known that the major irreversible toxicity of aminoglycosides is ototoxicity within the inner ear; it is the cochlear and vestibular sensory epithelium that is damaged with subsequent injury to the sensory cells and neurons, and this all causes permanent hearing loss (
8).
A major mechanism of the toxicity is apoptosis as a consequence of ROS over-production, interference with mitochondrial protein synthesis, stimulation of the N-methyl-D-aspartate (NMDA) receptors, and caspase-8 and 3 activation (
9,
10).
Among these processes, ROS give rise to activation of the caspase pathway as well as to direct damage to the cell membrane and cell DNA (
11). NMDA receptor antagonists, nitric oxide synthase inhibitors, caspase inhibitors and free radical scavengers have been demonstrated to protect against the aminoglycoside-induced vestibulotoxicity in experimental studies (
12).
Melatonin is a natural occurring compound with well-known antioxidant properties. In addition, the effects of melatonin on mitochondrial homeostasis have been discovered in the last decade. Numerous in vitro and in vivo studies have documented the actions of melatonin to protect cells from the cytotoxic activities of free radicals (
13,
14).
In this study, we investigated the effect of melatonin by analyzing cell survival and also the levels of the ROS and caspase-3 expressions in gentamicin-induced hair cell death. For the results, the number of utricular hair cells decreased in the gentamicin-treated groups and the survival of hair cells was enhanced when melatonin was added. Moreover, melatonin had an anti-oxidant effect in a dose-dependent manner, and hair cell death was decreasing in the range of the melatonin concentration of 10-100 µM. These results mean that melatonin prevents utricular hair cells from gentamicin-induced apoptosis.
Numerous clinical studies concerned with melatonin have recently been performed since 1993 and they have confirmed the action of melatonin as a free radical scavenger. In this study, we could confirm the direct and indirect anti-oxidant effects of melatonin.
Caspase-3 is an important protein for the apoptosis of utricular hair cells because it processes caspases further downstream in the pathway. It has been identified as a key mediator of apoptosis in mammalian cells. It cleaves and activates caspases 6, 7, and 9, and the protein itself is processed by caspases 8, 9, and 10 (
15,
16). Activation of the upstream initiator caspases is related to caspases 8 and 9, and this activates downstream effector caspases such as caspase-3. The sequential activation of caspases plays a central role in the execution-phase of cell apoptosis (
17,
18). Gentamicin causes an increase of both ROS and pro-apoptotic protein Bax in utricular hair cells, whereas the anti-apoptotic protein Bcl-2 is decreased (
19). Melatonin appears to have antioxidant effects to decrease the expression of Bax and increase the expression of Bcl-2.
For determining the expression of caspase-3 with using the caspase-3 substrate, we found that group G showed the strongest caspase-3 expression. Melatonin treatment (genamicin 1 mM plus melatonin 50 µM) attenuated the activation of caspase-3. This study demonstrated that melatonin could suppress the apoptosis induced by gentamicin in the utricular hair cells. We also demonstrated that one of the protective effects of melatonin was the attenuation of the caspase-3 expression.
We have shown in this study that melatonin is both a potent free radical scavenger and an anti-apoptotic agent in utricular hair cells.
Go to :












 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download