Abstract
Objectives
To evaluate characteristic computed tomography (CT) and magnetic resonance (MR) imaging findings of granulocytic sarcomas of the head and neck.
Methods
The CT (n=11) and MR (n=1) images obtained from 11 patients (7 males and 4 females; mean age, 23.5 yr; age range, 1 to 69 yr) with histologically-proven granulocytic sarcomas of the head and neck were retrospectively reviewed. Histological confirmation was done by bone marrow biopsy in 9 patients, and/or local biopsy in 4 patients. The imaging findings were analyzed with particular attention to location, size, shape, margin, bone destruction, internal architecture, pattern and degree of enhancement, and multiplicity of the lesions.
Results
The masses were most commonly located in the orbital cavity (n=8); other locations included lymph nodes (n=5) and palatine/pharyngeal/lingual tonsils (n=3). The mass sizes varied from a mean diameter of 1.3 to 5.8 cm (average, 2.6 cm). Multiple lesions were found in 6 patients. The shapes of the tumors were ovoid in 12 patients and irregular in 4 patients. Most lesions had poorly-defined margins (13/16) and invaded adjacent bony structures (5/16). On the pre-contrast CT images, the masses were iso- (5/8) or low-density (3/8) in comparison with muscle. The MRI, which was obtained in one patient in this study, showed that the mass was iso-signal intensity on T1-weighted images and iso-signal intensity on T2-weighted images compared to the gray matter of the brain. On the post-contrast CT images, there was homogenesous (n=12) or heterogeneous (n=4) enhancement, with mild (n=10), moderate (n=4), and marked (n=2) enhancement in the solid portions of the lesions.
Granulocytic sarcoma, also known as chloroma or extramedullary myeloblastoma, is a rare solid tumor comprised of primitive precursors of the granulocytic series of white blood cells, which include myeloblasts, promyelocytes, and myelocytes (1). Although usually associated with systemic disease in acute myeloid leukemia, granulocytic sarcoma may herald leukemic transformation in myelodysplastic disorders or disease, including chronic myeloid leukemia, polycythemia rubra vera, myelofibrosis, and chronic eosinophilic leukemia (2).
Originally described in 1811 by Burns, the term "chloroma" was first used in 1853 to refer to the green color of the tumor caused by the high content of myeloperoxidase (2). However, because up to 30% of these tumors can be white, gray, or brown, Rappaport (3) renamed them granulocytic sarcomas in 1966.
Radiologic descriptions of granulocytic sarcomas in the head and neck area are rare in the medical literature (4, 5), and consist mainly of case reports (6-8). The aim of this article was to determine the most common and/or characteristic imaging findings of granulocytic sarcomas in the head and neck area.
We reviewed the computed tomography (CT) (n=11) and magnetic resonance maging (MRI) (n=1) images obtained from 11 patients with granulocytic sarcomas in the head and neck. The patients were recruited after a review of the medical records from 3 tertiary referral academic centers during the past 7 yr. Complete hematologic investigations, including bone marrow aspirations, were performed in 9 of the 11 patients. In four cases, a direct histologic diagnosis of granulocytic sarcoma was obtained through biopsies of the affected head and neck sites. The diagnosis of patient was histologically-confirmed by both bone marrow and lymph node biopsies. In the other patients, we regarded the head and neck mass as a granulocytic sarcoma because of the prompt reduction in mass size after chemotherapy or irradiation. The specific subtype diagnoses of the 11 patients included acute myeloid leukemia in 8 patients, chronic myeloid leukemia in 1 patient, and an unknown myeloid tumor in 2 patients. There were 7 males and 4 females, 1-69 yr of age (mean age, 23.5 yr) who comprised the study cohort.
CT scanning was performed in 11 patients, and magnetic resonance (MR) imaging was performed in 1 patient. Only one patient underwent both CT and MR imaging examinations.
CT scans were obtained in the axial plane by using various models of a helical CT scanner with sections 2.5-3.75 mm in thickness. All 11 patients underwent CT scans after the intravenous administration of iodinated contrast material; of these 11 patients, pre=contrast CT scans were available in 6.
The MR imaging examination was performed on a 1.5T scanner (Signa Advantage; GE Healthcare, Milwaukee, WI, USA) using a head coil. The pre-contrast T1-weighted spin-echo images (TR/TE/NEX [483 ms/12 ms/1]) and T2-weighted fast spin-echo images (TR/TE/NEX [3,716 ms/102 ms/1]) with and without fat saturation were obtained, followed by contrast-enhanced spin-echo images with fat saturation after the intravenous injection of 0.1 mmol/kg of gadopentetate dimeglumine. Images were obtained in at least 2 planes with sections 3-4 mm in thickness, a 0-0.4 mm intersection gap, a 256×192 matrix, and a 22 cm field of view.
Two experienced radiologists with 4 and 14 yr of clinical experience retrospectively reviewed all of the CT and MR images for consensus. We investigated the CT and MR imaging characteristics with emphasis on the location and extent, size, shape, margin, adjacent bone involvement, internal architecture, pattern and degree of enhancement, and multiplicity of the lesions. The size of the lesion was measured in 3 dimensions. The margin of the lesion was classified as well- or ill-defined. The attenuation of the lesion on pre-contrast CT scans was compared with that of adjacent muscle to discern the internal architecture. The signal intensity of the lesion on T1- and T2-weighted MR images was compared with that of the cerebral cortex. On post-contrast CT and MR images, the pattern of enhancement at the solid portions of the lesion was categorized as homogeneous or heterogeneous. The degree of enhancement was subjectively assessed as mild, moderate, or marked. In the cases involving multiple lymph nodes or multiple tonsils, the largest among the lymph nodes or tonsils was evaluated.
The CT and MR imaging features of 11 cases of granulocytic sarcomas of the head and neck are summarized in Table 1.
Greater than 16 head and neck tumors were found in 11 patients. Multiple enlarged lymph nodes or tonsils were counted as one. All lesions were well-defined (n=13) or ill-defined (n=3) aggressive soft tissue masses in various locations of the head and neck, ranging in size from 1.3-6.8 cm (mean, 2.6 cm). The centers of the lesions appeared to be located in the orbits (n=8; Figs. 1 and 2), lymph nodes (n=5; Figs. 3 and 4), and palatine/pharyngeal/lingual tonsils (n=3). In one patient with bilateral orbital masses, the lesions contiguously extended to the adjacent maxillary sinuses. Multiple lesions were found in six patients, and involved the orbits and/ or lymph nodes and/or tonsils (Fig. 4). The shapes of the tumors (n=16) were ovoid (Fig. 4) in 12 patients and irregular (Fig. 1) in 4 patients. Five orbital masses were accompanied by adjacent bone destruction (Fig. 2) in four patients.
Compared with the adjacent muscle, the lesions (n=8) seen on pre-contrast CT scans obtained in six patients were iso-attenuated in five and hypo-attenuated in three. Compared with the cerebral cortex, the lesions had an iso-signal intensity on both the T1- and T2- weighted images. On the post-contrast CT images, there was homogenesous (n=12) or heterogeneous (n=4) enhancement with mild (n=10), moderate (n=4), and marked (n=2) enhancement in the solid portions of the lesions. On the post-contrast MR images, the lesion showed homogeneous marked enhancement (Fig. 2).
Granulocytic sarcomas have been observed in patients with acute myelogenous leukemia, chronic myelogenous leukemia, and other myeloproliferative diseases, such as myelofibrosis with myeloid metaplasia, hypereosinophilic syndrome, or polycythemia vera (1).
The most common setting for granulocytic sarcoma is disease progression in acute myeloid leukemia (73% of the patients). Granulocytic sarcoma lesions are multiple and solid, recurring at different sites in nearly 50% of the patients. In the study by Ooi et al. (4), the central nervous system, subcutaneous tissues, and genitourinary system were the most common sites of disease, accounting for nearly 52% of all lesions. In contrast, in the study by Pui et al. (5), the skin and orbit were the most common sites.
The orbit, skull, and epidural spaces are the preferred sites of head and neck involvement because granulocytic sarcomas arise in the bone marrow and traverse the Haversian canals to reach the periosteum. Rare lesions have been reported in the maxilla, soft palate, paranasal sinus, nasopharynx, salivary gland, scalp, and temporal bone (1, 5-8).
In our cases, the granulocytic sarcomas were most commonly found in the orbit (8 masses in 7 patients), and most of the lesions (5 of 8 tumors) were accompanied by adjacent bone marrow involvement. The incidence of bone marrow involvement is controversial between authors. In the review article by Guermazi (1), bone marrow involvement was common. In the study by Ooi, et al. (4), however, bone involvement with actual bone erosion was rare, and the most of the lesions arose either in solid organs or soft tissues.
Granulocytic sarcomas of the orbit often precede the blast phase of systemic disease and can represent the initial manifestation of leukemia (1). Usually, orbital masses arise intraconally or extraconally, are often bilateral, homogeneous, well-defined, and mold to the bone and contiguous structures (1), characteristics which are quite similar to our findings reported herein.
As reported in previous studies, the soft tissue granulocytic sarcomas are known as iso-dense or hyper-dense to brain or muscle on unenhanced CT, hypo-intense or iso-intense on T1-weighted MR images, heterogeneously iso-intense or hyper-intense on T2-weighted MR images, and homogeneously enhance after injection of contrast medium (1). These imaging findings agree with our study.
In our study, most of the lesions showed mild homogeneous enhancement. Some of the lesions had heterogeneous enhancing or non-enhancing areas which appeared to being caused by necrosis. In cases with multiple enlarged lymph nodes, some of the lymph nodes were heterogeneously-enhanced, but the other lymph nodes were homogeneously-enhanced (Fig. 3).
Soft-tissue masses arising in patients with myelogenous leukemia may have several possible causes, including abscesses or hematomas. Granulocytic sarcomas are not common, but they are important to recognize because they respond more favorably to focal irradiation than to systemic chemotherapy. CT and MR imaging are useful in revealing granulocytic sarcomas, delineating the extent for radiation therapy, planning needle biopsy, and evaluating therapeutic response (1).
Signs strongly suggestive of granulocytic sarcomas are multiple, enhancing, solid masses occurring at different sites and time points during the course of disease in a patient with either acute myeloid leukemia or myeloproliferative or myelodysplastic disorders (4). In our study, the majority of the cases (6/11) had multiple lesions in different locations. Although the imaging appearance is non-specific, granulocytic sarcomas should be strongly considered whenever a mass is discovered in a patient with myelogenous leukemia (1). In isolation, granulocytic sarcoma masses
may be difficult to differentiate from other focal malignant tumors; however, the clinical presentation, such as multiple sites of tumors, recurrent nature, and underlying hematologic malignancies, can facilitate the proper diagnosis (4).
The limitation of our study was the small number of localized tumor biopsies. Usually, the diagnosis of leukemia is achieved by bone marrow biopsy. In only four cases of the patients presented herein were the granulocytic sarcomas confirmed by local biopsies of soft tissue tumors. In the usual clinical setting of leukemia, the presumed granulocytic sarcomas are managed with prompt chemotherapy and/or irradiation, rather than confirmation by tissue biopsy. In our cases, we regarded the head and neck masses as granulocytic sarcomas because the masses decreased in size immediately after chemotherapy or irradiation (Fig. 4).
In our cases, the masses were located in the orbit with/without extension to adjacent tissues (maxillary sinus, sphenoid bone, and temple soft tissue), tonsils, or lymph nodes. In the cases of the orbital masses, especially which were located in superolateral aspect of the orbits, we suggest that the imaging findings are sufficient to make the diagnosis of granulocytic sarcoma. In the lymph nodes, enlarged lymph node size with loss of normal ovoid shape and/or loss of hilar vascular opacity and/or loss of hilar fat opacity, may be a sign of malignancy (9). In the tonsilar mass in adenoid or palatine tonsils, enlarged size with loss of thin striations seen in lymphoid hypertrophy may be a sign of malignant tumor infiltration (10). When we encounter a head and neck mass, especially in a patient presumed to have leukemia, these imaging findings will be helpful to secure an early diagnosis of granulocytic sarcoma.
Although rare, granulocytic sarcomas arise in various anatomic locations in the head and neck area, most commonly in the orbit with the form of a well-demarcated, mildly- and homogenously-enhancing mass with adjacent bony invasion. Frequently, granulocytic sarcomas have multiple lesions involving the orbit and/or tonsils and/or lymph nodes. Although the imaging findings are non-specific, granulocytic sarcoma should be included in the differential diagnosis of an orbital mass with bone destruction or tumors showing multiplicity in the head and neck area.
References
1. Guermazi A, Feger C, Rousselot P, Merad M, Benchaib N, Bourrier P, et al. Granulocytic sarcoma (chloroma): imaging findings in adults and children. AJR Am J Roentgenol. 2002; 2. 178(2):319–325. PMID: 11804886.
2. Liu PI, Ishimaru T, McGregor DH, Okada H, Steer A. Autopsy study of granulocytic sarcoma (chloroma) in patients with myelogenous leukemia, Hiroshima-Nagasaki 1949-1969. Cancer. 1973; 4. 31(4):948–955. PMID: 4513297.

3. Rappaport H. Atlas of tumor pathology, section 3, fascicle 8: tumors of the hematopoeitic system. 1966. Washington (DC): Armed Forces Institute of Pathology.
4. Ooi GC, Chim CS, Khong PL, Au WY, Lie AK, Tsang KW, et al. Radiologic manifestations of granulocytic sarcoma in adult leukemia. AJR Am J Roentgenol. 2001; 6. 176(6):1427–1431. PMID: 11373207.

5. Pui MH, Fletcher BD, Langston JW. Granulocytic sarcoma in childhood leukemia: imaging features. Radiology. 1994; 3. 190(3):698–702. PMID: 8115614.

6. Lee YH, Lee NJ, Choi EJ, Kim JH. Granulocytic sarcoma (chloroma) presenting as a lateral neck mass: initial manifestation of leukemia: a case report. Eur Arch Otorhinolaryngol. 2006; 1. 263(1):16–18. PMID: 16205903.

7. Lee B, Fatterpekar GM, Kim W, Som PM. Granulocytic sarcoma of the temporal bone. AJNR Am J Neuroradiol. 2002; 10. 23(9):1497–1499. PMID: 12372738.
8. Nayak DR, Balakrishnan R, Raj G, Pillai S, Rao L, Manohar C. Granulocytic sarcoma of the head and neck: a case report. Am J Otolaryngol. 2001; Jan–Feb. 22(1):80–83. PMID: 11172221.

9. Som PM, Brandwein MS. Som PM, Curtin DH, editors. Lymph Nodes. Head and neck imaging. 2003. 4th ed. St. Louis (MO): Mosby;p. 1805–2003.
10. Mukherji SK. Som PM, Curtin DH, editors. Pharynx. Head and neck imaging. 2003. 4th ed. St. Louis (MO): Mosby;p. 1465–1520.
Fig. 1
A 27-yr-old woman with a 1-yr history of acute myeloid leukemia presented with painful, reddening of the right orbit. (A) Post-contrast axial CT image shows homogeneously-enhancing soft tissue mass with irregular shape in intraconal and extraconal spaces of the right orbit. (B) Post-contrast coronal CT image shows large enhancing mass without adjacent bone involvement in the right orbit.
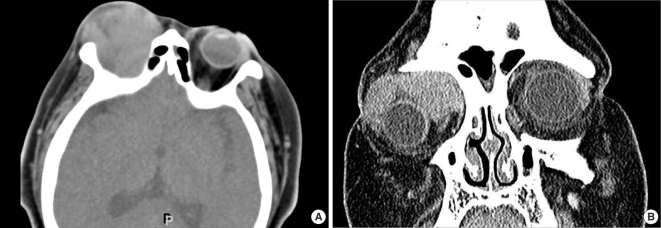
Fig. 2
A 69-yr-old man with a 7-yr history of chronic myeloid leukemia presented with left orbital pain. (A) T2-weighted axial image shows an iso-signal intensity mass (arrows) in the lateral side of left orbit. (B) T1-weighted axial image shows an iso-signal intensity mass (arrows). Adjacent bone marrow was involved by the tumor. (C) The mass is well-enhanced on the post-contrast T1-weighted coronal MR image (arrows). (D) Post-contrast coronal CT image shows the mass (arrows) involving the adjacent sphenoid bone and the intracranial area.
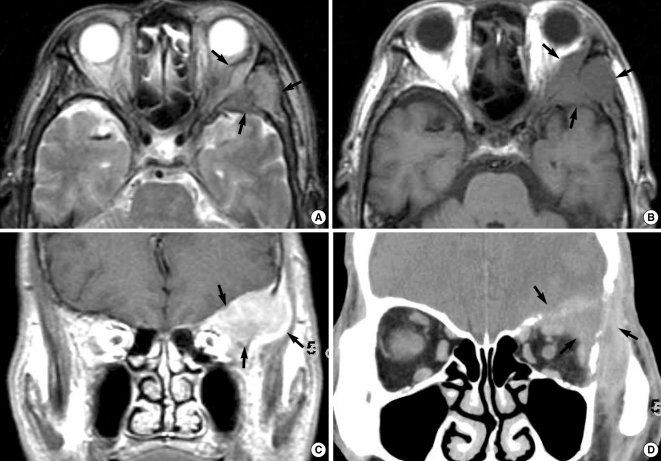
Fig. 3
An 11-yr-old boy with a 2-yr history of acute myeloid leukemia presented with cervical swelling. Post-contrast axial CT image shows multiple homogeneously-enhancing lymph nodes (white arrows) and lymph node with heterogeneous enhancement due to necrosis (black arrow) along the lateral cervical lymph node chains. The lymph node was shown to be a granulocytic sarcoma by local lymph node biopsy.
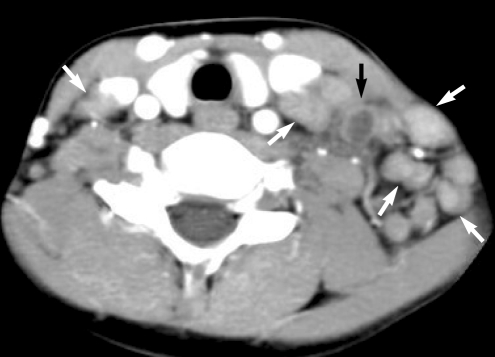
Fig. 4
A 16-yr-old man presented with a left neck mass as an initial manifestation of leukemia. (A) Post-contrast CT scan shows slightly enhancing mass in the superolateral peripheral space of the left orbit. (B) Post-contrast CT scan of the neck shows enlarged and enhancing lymph nodes (arrows) in the lower neck. (C) The lymph nodes are decreased in size on the CT image 7 days after induction chemotherapy.
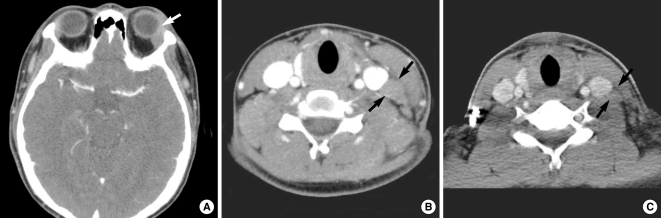
Table 1
Summary of clinical findings and CT and MR features of 11 patients with chloromas of the head and neck
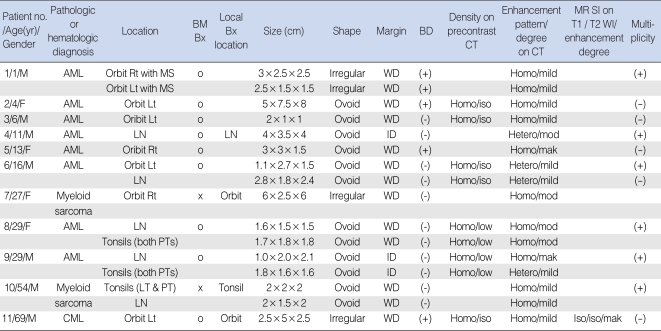
*Size was denoted as 3 dimensions; Margin was denoted as WD or ID; CT density of the lesion was compared with that of the adjacent muscle; MR signal intensity of the lesion was compared with that of the cerebral cortex.
BM Bx.: indicates bone marrow biopsy; Bx: biopsy; MS: maxillary sinus, AML: acute myeloid leukemia; CML: chronic myeloid leukemia; LN: lymph node, PT: palatine tonsil; LT: lingual tonsil; T1WI: T1-weighted image; T2WI: T2-weighted image; WD: well-defined; ID: ill-defined; BD: bone destruction; homo: homogeneous; hetero: heterogeneous; mod: moderate; mak: marked.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download