Abstract
Basaloid squamous cell carcinoma (BSCC) is often founded in the head and neck region. However, BSCC in the sinonasal tract is rare. We report here on the case of a 58-yr-old woman who presented with nasal obstruction and epistaxis. Computed tomography and examination of the nasal cavity revealed a tumor mass that originated from the right inferior turbinate with erosion of the nasal floor. The tumor that was attached to the inferior turbinate, the lateral nasal wall and the eroded right side hard palate, and so all this was resected. Histopathologic examination of the excised tumor confirmed BSCC in the nasal cavity. We report here on a nasal cavity BSCC that was treated with partial maxillectomy only.
Basaloid squamous cell carcinoma (BSCC) is considered a high-grade variant of squamous cell carcinoma that preferentially arises in the upper aerodigestive tract, i.e., the base of the tongue, the larynx and the hypopharynx. Most BSCCs are diagnosed at advanced clinical stages and they have an unfavorable prognosis because of the poor overall patient survival rates. There have been a few reported cases of sinonasal tract BSCCs. According to Lu et al. (1), less than 30 cases of sinonasal BSCC have been reported since Wain first described this as a distinct entity at 1986. Here we describe a patient who has nasal obstruction and frequent epistaxis due to nasal cavity BSCC.
A 58-yr-old woman presented with a several month history of epistaxis and right side nasal obstruction. She denied using tobacco or alcohol. Upon nasal examination, we noted a tumor mass that was attached to the right side inferior turbinate. The computed tomography (CT) scan revealed a tumor mass involving the right nasal cavity and the right nasal floor with erosion of bone (Fig. 1). All the other laboratory data was within normal limits.
An endoscopic biopsy was performed under local anesthesia. On the microscopic examination, the tumor was composed of closely packed solid lobules of basaloid cells with areas of comedo-type necrosis (Fig. 2A). Abundant intercellular hyaline globules and abrupt keratinizations were frequently seen in the nests of basaloid cells (Fig. 2B). Peripheral palisading of the nuclei was also seen (Fig. 2C). For the immunohistochemical staining, the tumor cells were positive for p63 (Fig. 3A) and high molecular weight cytokeratin (Fig. 3B) and they were negative for chromogranin and CD56 (Fig. 3C). The above histologic and immunohistochemical findings were consistent with basaloid squamous cell carcinoma. A metastatic workup, including brain CT and positron emission tomograohy (PET), was negative for signs of nodal involvement or metastases to other organs. The patient underwent right side partial maxillectomy with enbloc resection of the tumor. The surgical specimen included tumor attached to the right side inferior turbinate and hard palate. All the resection margins were clear. Histopathological examination of the excised lesion showed a 2.5×1.7×1.2 cm sized basaloid squamous cell carcinoma. No adjuvant chemotherapy or radiation was administered. The patient is in good health with no evidence of complication or recurrence seventeen months after surgery.
BSCC is a rare and aggressive variant of SCC that was first identified as a separate histopathologic entity by Wain and others (2). Since their report, there have been reports of BSCCs of the head and neck regions, such as the oral cavity, palate, the floor of mouth, nasopharynx, oropharynx and mastoid. Although this type of tumor is most commonly found in the head and neck region, BSCC in the nasal cavity is rare with less than 30 cases having been reported in the current literature (1).
Microscopically, BSCC can have a lobular, cord-like, cribriform, tubular, glandula-like or nest pattern, and the can be focally connected to the surface epithelium. The cells at the periphery of the lobules are often palisaded, with hyperchromatic nuclei and scant cytoplasm. The differential diagnosis of sinonasal BSCC includes small cell undifferentiated neuroendocrine carcinoma, adenoid cystic carcinoma, olfactory neuroblastoma and sinonasal undifferentiated carcinoma. Immunohistochemical staining can help make the differential diagnoses. BSCC is distinguished by positive staining for cytokeratin and epithelial membrane antigen, and negative staining for carcinoembryonic antigen and vimentin. Such neuroendocrine markers as synaptophysin and chromogranin are positive for small cell undifferentiated neuroendocrine carcinoma and olfactory neuroblastoma, while they are negative for BSCC. Adenoid cystic carcinoma shows greater reactivity for vimentin as compared with BSCC. Undifferentiated carcinoma is an anaplastic neoplasm that shows the absence of diagnostic markers.
It has been reported that BSCC often shows an aggressive biological behavior with a high incidence of cervical lymph node metastasis and distant metastasis (3). Raslan et al. (4). reported the incidence of neck node and distant metastasis in head and neck BSCC was 64% and 44%, respectively, with 38% mortality and a median survival of 17 months. Meanwhile, one study showed that among 20 patients with BSCC in the head and neck, 11 of the patients had no evidence of disease with at least 2 yr of follow-up and one patient is disease-free 8 yr after treatment (5). Although BSCC is an aggressive neoplasm, it is not uniformly fatal.
Because of the limited cases in the sinonasal tract, various treatment modalities have been tried in other reports. The generally recommended treatment options for BSCC of the head and neck includes complete surgical excision supplemented with radiotherapy. But in this case, the patient was treated with only surgery because all of the surgical margins were free of tumor and some of the other previous studies stressed that an early stage of tumor may be regarded as a different category and so this can be treated more conservatively (6). Our patient is under close observation and greater numbers of patients must be studied to recommend further therapy after surgery.
References
1. Lu SY, Eng HL, Huang CC, Chien CY, Lui CC, Lin JW. Basaloid squamous cell carcinoma of the sinonasal tract: report of two cases. Otolaryngol Head Neck Surg. 2006; 5. 134(5):883–885. PMID: 16647553.

2. Wain SL, Kier R, Vollmer RT, Bossen EH. Basaloid-squamous carcinoma of the tongue, hypopharynx, and larynx: report of 10 cases. Hum Pathol. 1986; 11. 17(11):1158–1166. PMID: 3770734.
3. Coppola D, Catalano E, Tang CK, Elfenbein IB, Harwick R, Mohr R. Basaloid squamous cell carcinoma of floor of mouth. Cancer. 1993; 10. 72(8):2299–2305. PMID: 7691390.

4. Raslan WF, Barnes L, Krause JR, Contis L, Killeen R, Kapadia SB. Basaloid squamous cell carcinoma of the head and neck: a clinicopathologic and flow cytometric study of 10 new cases with review of the English literature. Am J Otolaryngol. 1994; May–Jun. 15(3):204–211. PMID: 8024109.

5. Paulino AF, Singh B, Shah JP, Huvos AG. Basaloid squamous cell carcinoma of the head and neck. Laryngoscope. 2000; 9. 110(9):1479–1482. PMID: 10983946.
6. Sheen TS, Chang Y, Ko J, Wu C, Lee S. Basaloid squamous cell carcinoma of the larynx. Otolaryngol Head Neck Surg. 1999; 11. 121(5):647–650. PMID: 10547488.

Fig. 1
Coronal & sagittal CT of the paranasal sinus shows the right nasal cavity mass (arrows). The tumor mass showing focal enhancement with bone erosion at the inferior turbinate and hard palate. (A) Sagittal view. (B) Coronal view.

Fig. 2
Pathologic findings. (A) Irregular lobules of basaloid cells with comedo-type necrosis (arrow; H&E, ×40). (B) Abundant intercellular hyaline globules (white arrow) and multifocal keratinization (black arrow; H&E, ×200). (C) Nest of basaloid cells with peripheral palisading of the nuclei (arrows; H&E, ×400).
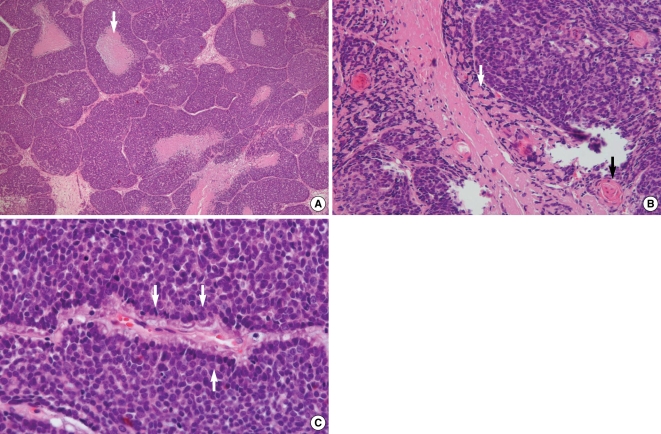
Fig. 3
Immunohistochemical findings showing the basaloid squamous cell features of the tumor cells (×400). (A) Nuclear immunoreactivity on the p63 staining with a brownish color (arrow). (B) Cytoplasmic and cytoplasmic membranous immunoreactivities on the high molecular weight cytokeratin staining with a brownish color (arrow). (C) No immunoreactivity on the chromogranin & CD56 staining.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download