INTRODUCTION
MATERIALS AND METHODS
Nasal polyp epithelial cell culture and activation with HDM
Co-culture of the epithelial cells and PBMCs
The intercellular adhesion molecule 1 (ICAM-1) expression of the nasal epithelial cells and the inhibition with ICAM-1 antibody
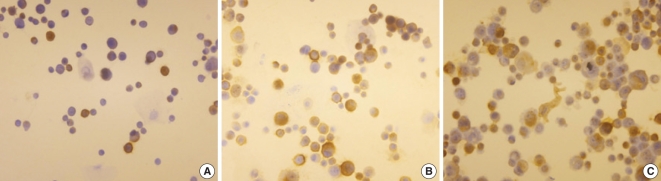 | Fig. 1The immunocytochemical findings of the nasal polyp epithelial cells treated with dermatophagoides farina (DF). (A) The nasal epithelial cells were not stimulated with DF. (B, C) The nasal epithelial cells were stimulated with 10 and 50 µg/mL of DF and the number of immune stained cells was increased. |
Statistical analysis
RESULTS
Activation of the nasal polyp epithelial cells with HDM
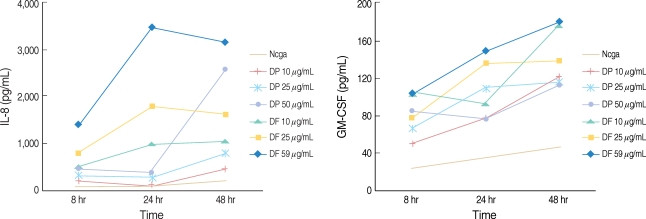 | Fig. 2Kinetic study of the interleukin-8 (IL-8) and granulocyte-macrophage colony stimulating factor production in the nasal polyp epithelial cells treated with house dust mites allergens. The cytokine productions were increased is a dose dependent manner.
DP: dermatophagoides pteronyssinus; DF: dermatophagoides farina; GM-CSF: granulocyte-macrophage colony stimulating factor.
|
Co-culture of epithelial cells and PBMCs
 | Fig. 3Production of cytokines from a co-cultrue of peripheral blood mononuclear cells and acativated nasal epithelial cells with house dust mites (HDM) allergens. Dermatophagoides farina (DF) enhanced the TNF-α and interleukin-5 production, and dermatophagoides pteronyssinus (DP) enhanced the tumor necrosis factor (TNF)-α production, but interferon (INF)-γ was not increased. The unit of the HDM extracts' concentration was µg/mL. Negative: the nasal epithelial cells were not stimulated by the HDM extracts. *P<0.05. |
The ICAM-1 expression of the nasal epithelial cells
 | Fig. 4The nasal epithelial cells' expression of intercellular adhesion molecule 1 (ICAM-1) mRNA (A) and cell membrane ICAM-1 (B). The ICAM-1 mRNA expression was increased after stimulation with dermatophagoides pteronyssinus. The cell membrane ICAM-1 expression was increasd after stimulation with dermatophagoides pteronyssinus (DP) and dermatophagoides farina (DF). The unit of the house dust mites extracts' concentration was µg/mL. Negative: the nasal epithelial cells were not stimulated by the house dust mites extracts. *P<0.05. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download