Introduction
The human upper airway is consisted with four anatomical levels; nasopharynx, velopharynx, glossopharynx, and laryngopharynx [
1]. Majority of obstructive sleep apnea syndrome (OSAS) patients are previously studied to have a narrowing of glossopharynx and hypopharynx. Uvulopalatopharyngoplasty (UPPP) is a widely accepted first-line surgical treatment method for resolving OSAS [
2]. However, in cases which UPPP fails to resolve OSAS, hypopharyngeal obstruction is accused to be the main reason [
3]. Therefore, the importance of manipulation of the base of tongue (BOT) is currently being issued [
4-
6]. For the reduction of glossopharyngeal narrowing, a midline glossectomy could be an option [
5]. In cases which hypopharyngeal narrowing is a problem, manipulation of BOT such as submucosal minimally invasive lingual excision, carbon dioxide laser midline glossectomy, hyoepiglottoplasty, and radiofrequency tissue reduction are accepted methods [
4,
5,
7]. In combination with or without UPPP, the effectiveness of BOT manipulation techniques has been proven in many previous studies [
5,
7].
In performing volumetric reduction in BOT, the hypoglossal/lingual artery neurovascular bundle (HLNVB) acts as a crucial anatomical hurdle [
8]. The lingual arteries are the most vulnerable structure among HLNVB upon performing BOT manipulation, which may result in fatal complications such as massive bleeding and immediate airway obstruction when injured [
9]. Many previous studies have focused on the course of the lingual artery and its spatial relation between adjacent anatomical landmarks [
6,
10-
13]. However, there were no single previous study discussing the difference of lingual artery and its relation with the tongue in OSAS patients compared with the normal population.
In Korea, no previous study arguing about the spatial relation between the lingual artery and the tongue in Korean population had ever been reported. With the necessity, the authors aimed to analyze the course of the lingual artery and its relation with the tongue in Korean male adult population.
Go to :

Subjects and Methods
Ethics statement
This study was approved by the Institutional Review Board of Asan Medical Center (Approval no. 2016-0348).
Patient (OSAS) group
From 2001 January to 2015 December, patients confirmed with OSAS by polysomnograph (PSG) in Asan Medical Center who underwent contrast-enhanced computed tomography (ceCT) or computed tomography angiography (CTA) of head and neck area for any occasion within one year prior or after PSG was included. Medical records, PSG data, and CT image of included patients were retrospectively reviewed.
Control group
The course of lingual artery and its spatial relation with the tongue were compared with the control group. Patients who had their ceCT or CTA image during the same study period mentioned above for any occasion (e.g.; acute tonsilitis, acute epiglottis, and etc.) were recruited, followed by randomized matching with the study group in terms of the age and the sex.
Exclusion criteria
In both OSAS group and control group, patients were excluded if they met the following criteria; 1) CT image of head and neck obtained from different protocols, 2) prior surgical history of naso-oro-hypo-pharyngeal area (e.g.; adenoidectomy, tonsillectomy, and etc.), 3) expected disruption of the natural course of the lingual artery (e.g.; abscess, tumors of pharyngeal region, and etc.), 4) female patients, and 5) patients under age of less than 20 years old.
The age was stratified upon every 10 years; the severity of obesity was classified according to the WHO 2000 guideline for Asia-Pacific population [
14]; the OSAS severity was stratified upon apnea-hypopnea index (AHI), as described in the 2015 American Academy of Sleep Medicine recommendations [
15].
Imaging modality
All OSAS patients and control subjects enrolled in the study have either received ceCT or CTA of head and neck area in the study period mentioned above.
CeCT was delivered by using a LightSpeed QX/i scanner (GE Medical Systems, Milwaukee, WI, USA) or Somatom Sensation 16 (Siemens Medical Solutions, Forchheim, Germany), and intravenous (IV) bolus injection of a nonionic iodinated contrast agent (140 mL) (Optiray; Mallinckrodt Pharmaceuticals, Dublin, Ireland) at a rate of 2.5 mL/s. Scanning was conducted using an 3 mm-thick sections, and a 20.9-cm field of view, 120-kV voltage, 200-mA tube current, and 256×256 matrix. Contiguous scans with 0.6 mm collimation were obtained at 3 mm intervals with no gap from the skull base to the upper chest for head and neck images. There were 70 seconds delay of the scan phase following IV contrast infusion, thereby enabling the identification of the arterial and the venous system of head and neck as well as the course of the lingual artery.
CTA was performed through Somatom Sensation 16. IV line was secured at the patients’ right arm, followed by a dual bolus IV infusion with a nonionic iodinated contrast media (60 mL) (Optiray) at a rate of 5 mL/s, in addition to saline infusion at a rate of 5 mL/s at the same time. Scanning was conducted using a 3 mm-thick sections, and a 20.9-cm field of view, 120-kV voltage, 200-mA tube current, and 256×256 matrix. Contiguous scans with 0.6 mm collimation were obtained at 3 mm intervals with no gap from the aortic arch to the vertex. Time delay from IV infusion to spiral scan was 14 seconds (monitoring delay; 10 seconds, followed by scan delay; 4 seconds). A Housefield unit of 100 was considered as arterial structure for the image reconstruction, thereby differentiating lingual artery from adjacent structures.
Metric evaluation of the tongue & the lingual artery
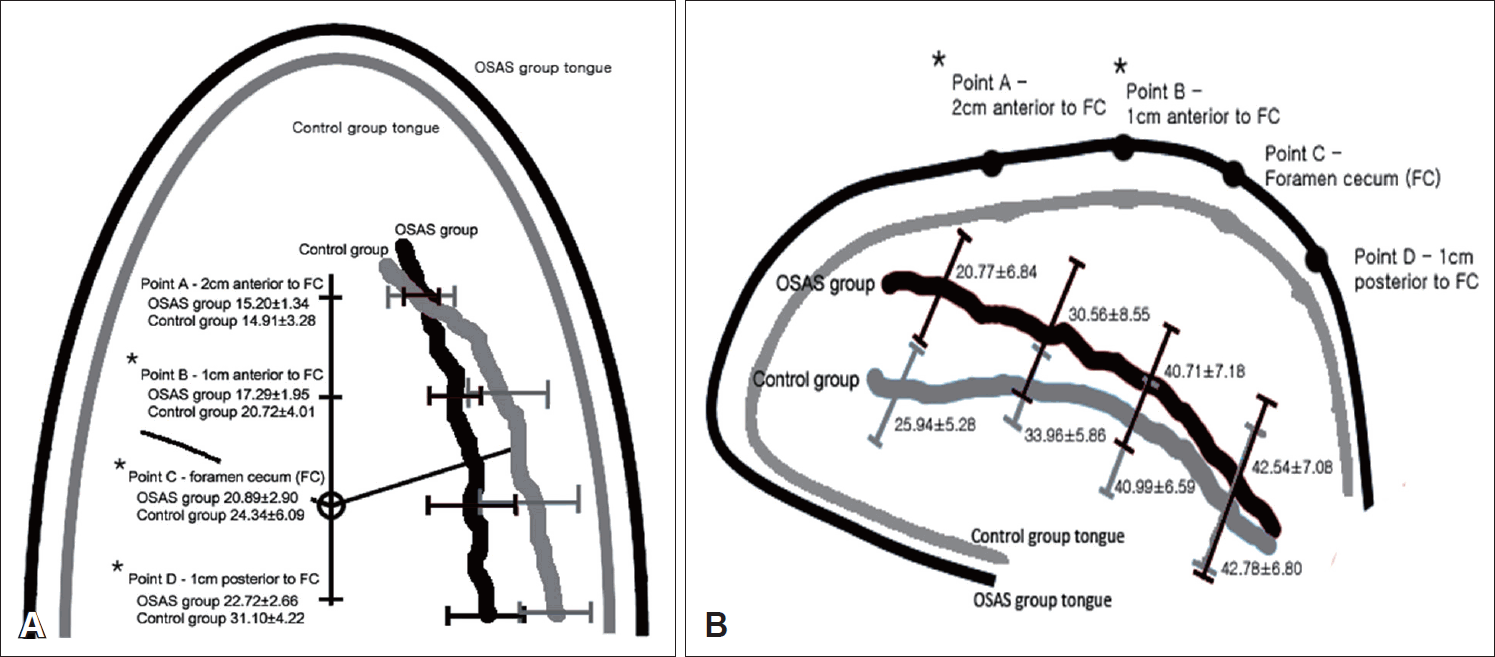
For the measurement of each distance parameters, four landmarks were set as the reference points. The foramen cecum (FC) linguae, a small midline depression on the lingual surface on the border dividing the anterior two-thirds (tongue tip and tongue body) of the tongue and the posterior aspect (base of the tongue) of the tongue, was set as the main landmark in the study. In the sagittal image shown in the obtained CT scan, the dimpling point on the border separating the tongue body and BOT was identified, and defined as “point C” (
Fig. 1). Three additional landmarks were defined as following detail; “point A,” located 2 cm anteriorly from point C on the lingual surface; “point B,” located 1 cm anteriorly from point C on the lingual surface; “point D” located 1 cm posteriorly from point C on the lingual surface. In all four points (point A-D), the depth of the lingual artery from the lingual surface of the tongue was measured on the sagittal image, and the width of lingual arteries bilaterally was measured on the axial image (
Fig. 2). In the middle anterior half portion of the tongue, the depth and the width of deep lingual artery was measured, and in the posterior half of the tongue, the depth and the width of lingual artery was evaluated. The thickness, width, and length of the tongue were also measured (
Fig. 3). Tongue thickness was measured as the distance from the lingual surface at FC level and mental spine on a sagittal image. The maximal width of the tongue shown on a coronal image was considered as the width of the tongue. The distance between the tip and the base of the tongue on a sagittal image was considered as the length of the tongue. All of the measurement process was done by a single specialist (M.J. Park), with the blinding of patients’ clinical information.
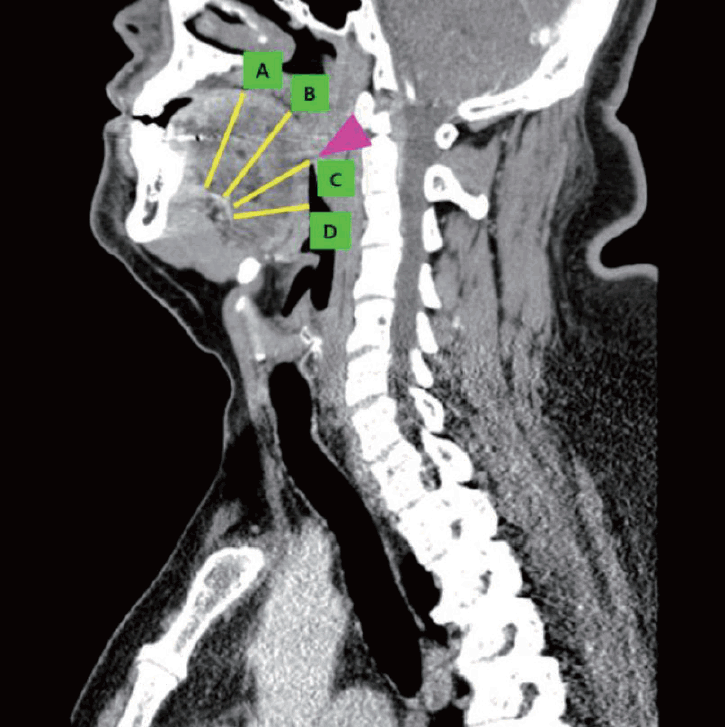 | Fig. 1.Establishment of the reference points. A contract-enhanced computed tomography sagittal image of a 33-year-old male patient confirmed with obstructive sleep apnea syndrome. The lingual artery is visualized, and the depth of the lingual artery from the lingual surface of the tongue at each four reference points (A–D) was measured. Arrowhead; FC. A: 2 cm anterior to FC. B: 1 cm anterior to FC. C: FC. D: 1 cm posterior to FC. FC, foramen cecum. 
|
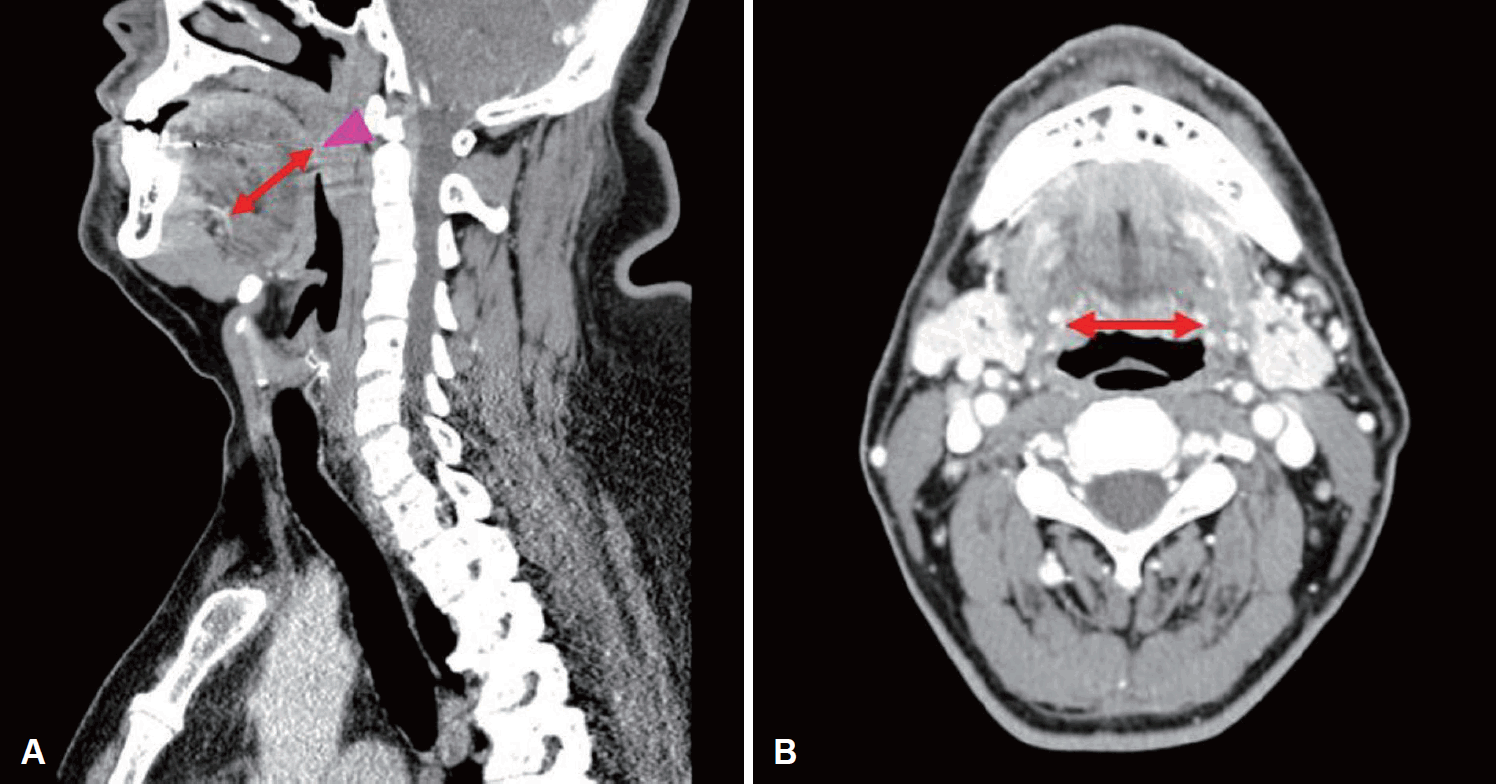 | Fig. 2.Measuring the depth and the width of the lingual artery. A contract-enhanced computed tomography sagittal image of a 33-year-old male patient confirmed with obstructive sleep apnea syndrome. A: On the sagittal image, the depth of the lingual artery (arrowline) was measured from the lingual surface of the tongue at the FC level (arrowhead). B: The width of the bilateral lingual arteries was measured at the level of FC (arrowline). FC, foramen cecum. 
|
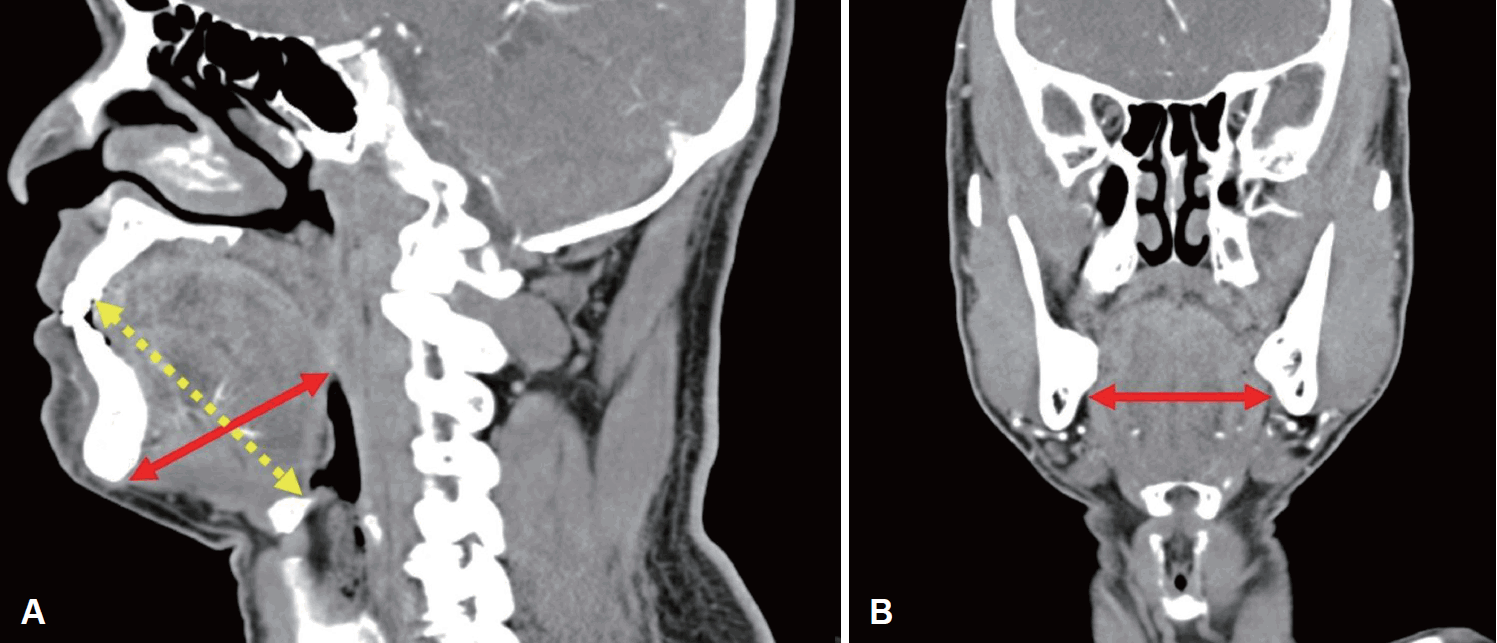 | Fig. 3.Measurement of the thickness, width, and length of the tongue. A: Tongue thickness was measured as the distance from the lingual surface at foramen cecum level and mental spine on a sagittal image (bold line arrows). The distance between the tip and the base of the tongue on a sagittal image was considered as the length of the tongue (arrow with dotted lines). B: The maximal width of the tongue shown on a coronal image was considered as the width of the tongue. 
|
PSG
Confirmation of OSAS was achieved based on full, standard PSG, delivered by RemLogic version 2.0 (Embla Systems Inc., Broomfield, CO, USA). A type 1 (standard, in-laboratory, technician-attended, overnight) protocol was employed. OSA was diagnosed when a patient had an AHI greater than 5 and symptoms of excessive daytime sleepiness, or an AHI greater than 15 regardless of daytime symptoms, in line with the 2015 American Academy of Sleep Medicine recommendations.15) The severity of OSA was judged from AHI data, and graded as mild OSA (5≤AHI<15/hr), moderate OSA (15≤AHI<30/hr), and severe OSA (AHI>30/hr).
Statistical analysis
Statistical analysis was performed using SPSS version 22 software (IBM Corp., Armonk, NY, USA). Comparison of demographical features and distance parameters measured at each four reference points between the OSAS and the control group was done by using the student’s t-test. Analysis of variance method was used for the analysis for the difference of each distance parameters in the subgroups of OSAS patients categorized by their obesity level, degree of OSAS, and the age. A Kolmogorov-Smirnov test was applied to determine whether each parameter showed a normal distribution. A p value less than 0.05 was considered as statistically significant.
Go to :

Discussion
Currently, many surgeons accept surgical approach for the correction OSAS when a conservative method (e.g.; weight loss, continuous positive airway pressure device, and etc.) have failed to achieve therapeutic target [
16]. UPPP is a powerful surgical method to resolve velopharyngeal obstruction, however has its demerit in cases which glossopharyngeal obstruction plays main role for the upper airway narrowing [
2,
3]. Manipulation of BOT in combination with UPPP or exclusively is an effective surgical modality for resolving hypopharyngeal narrowing, having its efficacies proven in many previous studies [
4,
5,
7]. When manipulating the BOT, protection of the lingual artery as well as other components in HLNVB should come as the first priority as well as effective volumetric reduction of the BOT in the same time. Injury to the lingual artery could result in serious complications such as perioperative bleeding, hematoma, profuse blood aspiration, and pseudoaneurysm thereby obstructing the upper and lower airway immediately after or during the surgery [
6,
8,
10,
17]. Therefore, the importance of precise understanding of the lingual artery precisely in OSAS patients cannot be overemphasized for the surgeon.
The lingual artery is a major artery branching out from the external carotid artery, and comes along as in the form of neurovascular bundle from the base of the tongue. HLNVB consists of lingual arteries, hypoglossal nerves, lingual nerves, and the lingual veins [
8]. Posteriorly to circumvallate papillae, the lingual artery branches the dorsal lingual artery vertically towards to the dorsal surface of the tongue. At the level of the middle portion of the tongue, the lingual artery is divided into two minor arteries; the deep lingual artery located above the lingual frenulum; the sublingual artery located below the lingual frenulum, forming abundant vascular anastomosis on hypoglossal floor [
18]. Lopez, et al. [
12] have claimed three territories of the tongue by vascular supply; the dorsal portion supplied by the deep lingual artery; the ventral portion supplied by the sublingual artery; the basal portion supplied by the dorsal artery. When performing the functional surgery of the tongue, ventral portion (below lingual frenulum) are rarely manipulated, and the deep lingual artery is a relatively well reflected structure on the dorsal tongue. In contrary, the posterior to middle third portion of the tongue and the hypoglossal floor are considered as the “restrictive zone” when performing the functional surgery of the tongue, thereby making the dorsal lingual artery and post half of the lingual artery the most important structure among the course of the lingual artery [
10,
11].
Previously, attempts to identify the course of the lingual artery and its relations within the tongue were done by many studies [
8,
12,
13,
18-
21]. The lingual artery was identified in ceCT images and in cadavers, therefore the depth and the width of the both lingual arteries were reported by scholars in Turkey, China and North America. Hou, et al. [
11] have proposed the “V zone” for the safe surgery of the tongue in OSAS patients, and Wu, et al. [
13] have analyzed the difference in the course of the lingual artery by the positional change of the tongue in Chinese population, and Cohen, et al. [
10] and Ibrahim, et al. [
21] have proven changes in the depth and the width of both lingual arteries by changes in the tongue position when retracted by a Feyh-Kastenbauer retractor, as if it was done in the setting for the transoral robotic surgery (TORS). Recently, Chang, et al. [
17] prosed an intraoperative ultrasound imaging during TORS, which resulted in faster, safe, and more efficient surgical outcome. In 2021, Gualtieri, et al. [
20] reported a new landmark on the ventral surface of the oropharynx to identify the lingual artery in the setting of a transoral surgery with resected head and neck specimen.
For the accurate comparison of the metric parameters between each study patients, a precise setting of each reference points was the single most important factor to consider upon study design. FC, located at the lingual surface of posterior one-third of the tongue, is an important landmark upon performing functional tongue surgeries. The feasibility of FC as a landmark for the evaluation of the lingual artery and the tongue had been validated by many studies over many decades [
6,
11-
13,
18,
20,
21]. Therefore, we have set FC as the main reference point, and three additional reference points were set as 2 cm anteriorly, 1 cm anteriorly, and 1 cm posteriorly to FC on the lingual surface. The reason that we have set each additional reference points on the lingual surface of the tongue is for easier orientation upon performing the surgery of the tongue. Clinically, it is the lingual surface of the tongue, not the lingual artery itself viewed by the surgeon when it comes to the surgical manipulation of the tongue [
20]. By identifying the FC on the lingual surface, the surgeon might easily get oriented of the spatial relation between the tongue and the lingual artery beneath the lingual surface, therefore lessening the rate of complications related to the damage of HLNVB. However, the exact location of FC is not easily identified on CT image, therefore we have set the FC as the point at one-third of the middle and posterior junction of the tongue in CT image exactly the same way as Wu, et al. [
13] have published.
Although the protocol for CT scan is different from the study conducted by Wu, et al. [
13] in China, we have compared our results side by side with their study. In patients confirmed with OSAS by PSG, both studies showed similar width of the lingual artery (20.9 mm in our study and 20.1 mm in Wu’s study), however showed deeper course of the lingual artery in our study (30.7 mm in ours and 27.9 mm in Wu’s) at the level of FC. The metric evaluation of the tongue showed slightly thicker tongue (58.7 mm in ours and 55.9 mm in Wu’s), however shorter tongue (74.9 mm in ours and 83.5 mm in Wu’s) in our study. A cadaveric study from China have revealed the safety margin for midline glossectomy as 7 mm lateral from the midline and 10 mm deep from the lingual surface at FC level [
22]. However, it should be reminded that these results were delivered from cadavers, not OSAS patients who tend to be more obese, and the cadaveric tissues were fixed which makes fatty component have shrink, thereby altering the vascular structures within the tongue.
The abnormal craniofacial anatomy in adult OSAS patients compared with normal population were previously studied by many researchers over a long period [
23-
25]. Metric analysis on the narrowing of upper airway due to shortening of bony structures and enlarged oropharyngeal structures such as palatine tonsil, BOT, and the tongue itself on physical examination and CT image were previously reported in many studies [
23,
24]. In addition, it had been argued patients with higher BMI tend to have more enlarged tongue with more abundant fat tissues in oropharyngeal structures, and these relationships were especially prevalent in Chinese population [
19,
25]. Similarly, our results showed the age-matched pairs of OSAS group had a thicker, wider, and longer length of the tongue compared with control, however these differences were no longer seen in BMI and age matched pairs (
Table 1). Interestingly, the relative depth and width of the lingual arteries showed some significant differences in few reference points between the OSAS group and the control group. However, it should be considered that the OSAS group showed significant wider, thicker, and longer size of the tongue. Therefore, although some significant differences in the depth and the width of the lingual artery were shown in some reference points, it cannot be straightforwardly concluded that the course of the lingual artery is shallower in the anterior half of the tongue. Data shown in
Table 2 shows no differences according to the level of obesity, age, and severity of OSAS were significantly related with the course of the lingual artery in both OSAS and control groups, supporting the fact that OSAS itself is an independent factor for shallower and narrower course of the lingual artery in the tongue, regardless of OSAS severity, age, and degree of obesity. The authors believe that these differences in the course of the lingual artery in the OSAS group and the control group are a result of the size difference of the tongue, however additional studies must be conducted to clear out this hypothesis.
Although we tried our best effort to minimize any bias and errors in the design of the study, we acknowledge some limitations as in following details. First, due to the fact that this study was a retrospective study, only one female OSAS patient was compatible for the inclusion criteria in the study period. To avoid any bias caused by the gender difference, we have excluded the female patient. Even though OSAS affects male population in Korea predominantly, it should be reminded that our data only represents the Korean male adult population. Second, in Wu [
13] and Hou’s [
11] study, measurement of the lingual artery shown on CT image was done in a similar way with our study. In their study, CTA was performed in all subjects, exclusively enhancing only the arteries whereas we have recruited patients CTA in addition with ceCT of the head and neck area which shows not only arterial structures but venous structures as well. Anatomically, the lingual artery courses close with the lingual vein, forming the HLNVB. Upon performing the surgery of the tongue, not only the lingual artery but also the HLNVB itself should be secured from injury, therefore we planned to include patients with ceCT image as well. Third, there is no evidence that the control group in our study does not possess OSAS, since no PSG was performed on these patients. Fourth, the majority of the control group had acute infectious condition of the palatine tonsil and epiglottis. Considering the fact that acute infection of those area can alter in mucosal swelling of the tongue base, there is a possibility that the lingual artery depth in control group were under or overestimated. Finally, due to the fact that our study is a retrospectively designed study, we were not able to clarify the spatial relation of the lingual artery and the tongue during various positional changes of the tongue, as suggested in publication by Lu, et al. [
26] Therefore, the authors acknowledge that the data provided in this paper is insufficient to provide a definite safety margin for the functional surgery in OSAS patients.
Despite some limitations, our study has its own unique clinical merits. First, sex and age-matched randomized normal population were recruited for the comparison. No significant difference of patients’ age was observed, in contrary to the fact that a significantly higher BMI of OSAS group was evident than the control which indicates the randomization and the selection of control was successfully handled. Second, to avoid any possible measurement bias, the clinical characteristics of each subject were blinded upon measuring the metric parameters. Third, considering the fact that the numbers of patients who receive sleep surgeries are increasing yearly in Korea, establishing a comprehensive knowledge on the spatial relation between the lingual artery and the tongue in Korean male patients is in need. Since we are the first to report these findings in Korean population, we believe our study could act as a “cornerstone” in future clinical practice for many surgeons.
The course of lingual artery and the spatial relation between the tongue differs in Korean male OSAS patients compared with matched normal population. In OSAS patients, the lingual artery showed shallower depth anterior to FC, and the length between both lingual arteries narrower distance around the level of FC, compared to normal population. Age, degree of obesity and PSG derived parameters were not significantly related with the spatial relation between the tongue and the lingual artery. In practicing the functional surgery of the tongue, it should be considered that the “safety area” of the OSAS patients is different from the normal population.
Go to :








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download