Introduction
Case
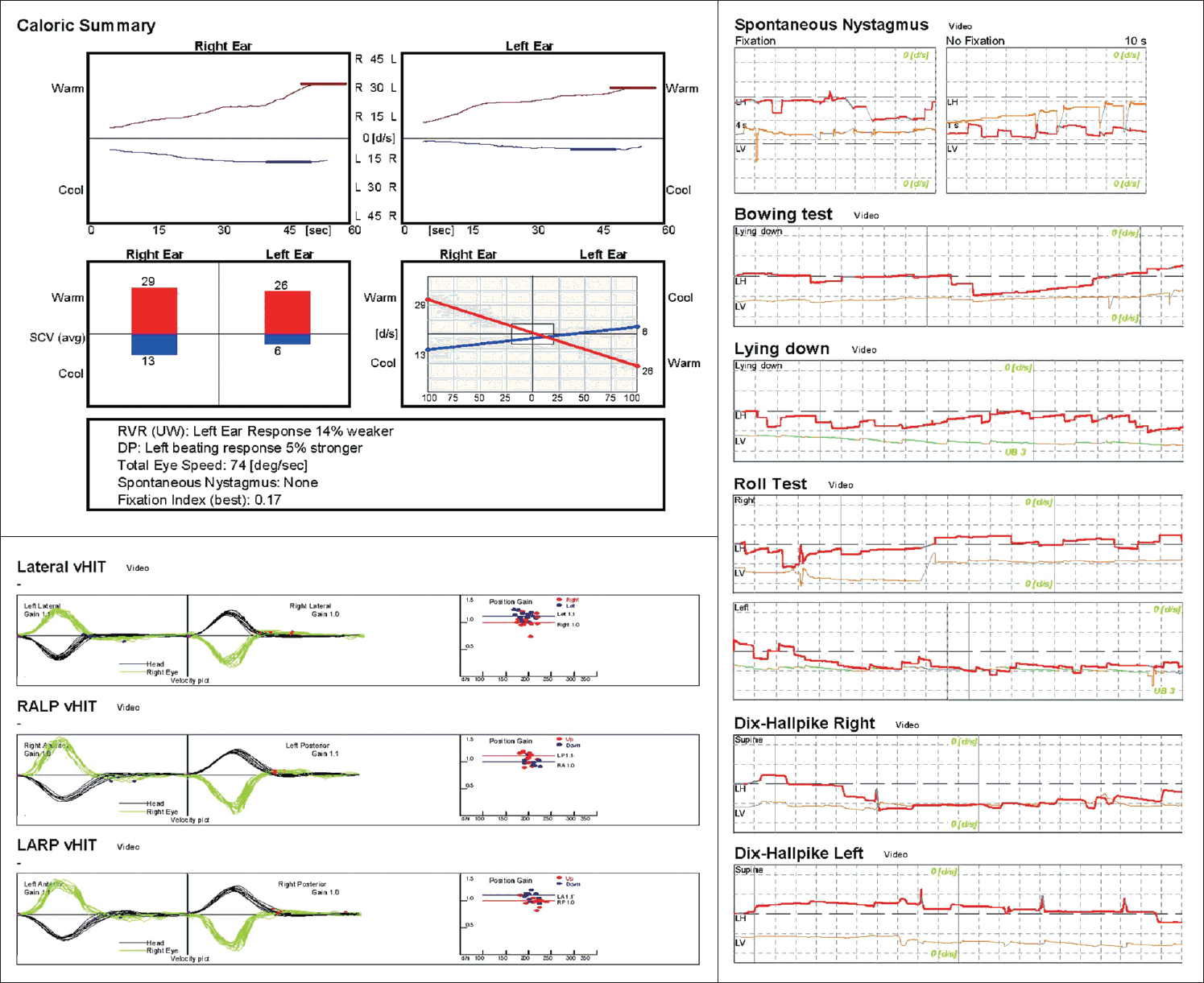 | Fig. 1.Results of the vestibular function test. The caloric response was within the normal range. Videonystagmography revealed no specific abnormal nystagmus, but subtle upbeat nystagmus was observed on the lean test, the left roll test, and both Dix-Hallpike tests, suggesting that the posterior canal may have been affected. The vHIT showed no abnormal saccades and the vestibulo-ocular reflex gains were normal. vHIT, video head impulse test. |
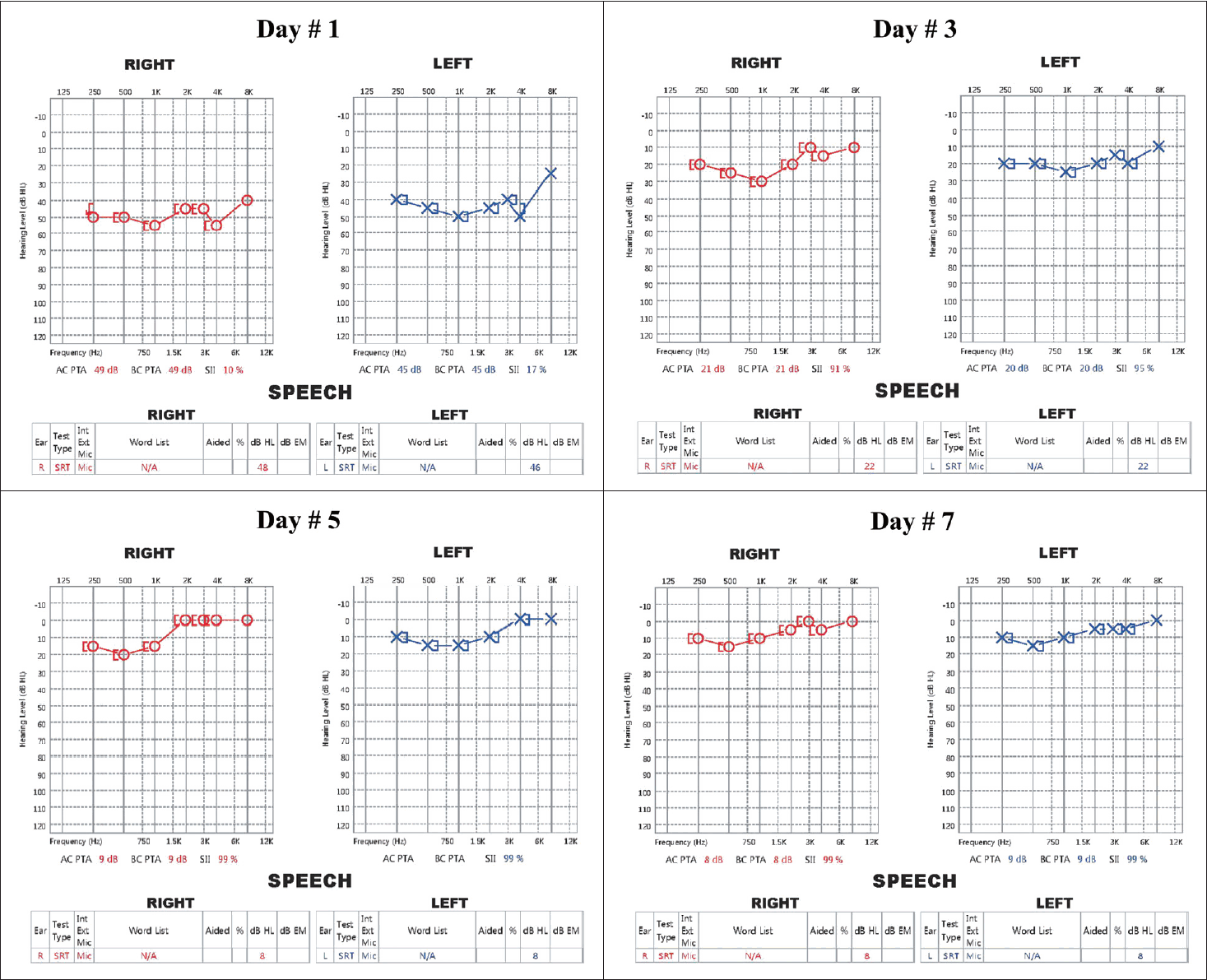 | Fig. 2.Sequential pure tone audiograms. The audiogram taken at the time of diagnosis revealed moderate, bilateral symmetrical SNHL. During treatment, the SNHL of both ears improved gradually as the general condition improved. The hearing improvements were symmetrical; the high frequencies recovered faster than the low frequencies. On day 7 of hospitalization, both ears evidenced near-normal hearing. SRT results did not differ significantly from the pure tone thresholds. SNHL, sensorineural hearing loss; SRT, speech recognition threshold. |
Table 1.
|
Patient |
Normal value | |||
|---|---|---|---|---|
| Initial | Day#3 | Day#7 | ||
| Age (year) | 28 | |||
| Sex | Female | |||
| Height (cm) | 150.6 | |||
| Weight (kg) | 47.8 | |||
| ABGA | ||||
| pH | 7.51 | 7.35-7.45 | ||
| pCO2 (mm Hg) | 34 | 32-46 | ||
| pO2 (mm Hg) | 108 | 80-90 | ||
| Bicarbonate (mmol/L) | 27 | 23-29 | ||
| O2 saturation (%) | 98.1 | 94.0-100.0 | ||
| Laboratory findings | ||||
| WBC (103/μL) | 30.60 | 19.30 | 12.43 | 4.00-10.00 |
| Neutrophil (%) | 82.6 | 78.2 | 76.8 | 50.0-75.0 |
| Hemoglobin (g/dL) | 8.4 | 8.8 | 9.5 | 12.0-16.0 |
| Platelet (103/μL) | 88* | 265 | 342 | 130-400 |
| ESR (mm/hr) | 117 | 90 | 61 | 0-20 |
| CRP (mg/L) | 96.3 | 31.9 | 9.8 | 0.0-5.0 |
| Na (mmol/L) | 123.2 | 132.7 | 136.5 | 136.0-146.0 |
| K (mmol/L) | 2.7 | 4.5 | 4.1 | 3.5-5.1 |
| Cl (mmol/L) | 81.4 | 100.2 | 106.0 | 101.0-109.0 |
| Bilirubin (mg/dL) | 2.38* | 1.08 | 0.72 | 0.30-1.20 |
| AST (U/L) | 70 | 49 | 28 | 1-37 |
| ALT (U/L) | 32 | 28 | 16 | 0-41 |
| BUN (mg/dL) | 14.6 | 10.3 | 8.9 | 8.0-20.0 |
| Creatinine (mg/dL) | 0.78 | 0.55 | 0.58 | 0.51-0.95 |
| Urine analysis | ||||
| Urine WBC (/HPF) | >100 | 50-99 | 20-29 | 0-4 |
| Urine bilirubin | 1+ | - | - | - |
| Urine urobilinogen | 2+ | - | - | - |
ABGA, arterial blood gases analysis; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; Na, sodium; K, potassium; Cl, chloride; AST, aspartate transaminase; ALT, alanine transaminase; BUN, blood urea nitrogen




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download