Abstract
Anaplastic thyroid cancers (ATC) are a rapid growing and highly lethal form of thyroid cancer. Distant metastases of ATC are detected in about half of patients. Clinically, it is very rare to metastasize to the kidney. The most common initial symptoms of ATC are a palpable neck mass and accompanying compression symptoms of the upper aerodigestive tract. A 68-year-old patient was referred with a huge renal mass, which was detected during the evaluation of abdominal pain. Left total nephrectomy was performed. Histologically, the kidney tumor was a metastatic anaplastic thyroid carcinoma. To our knowledge, it is the first case of occult ATC presenting as a huge renal mass.
Occult primary tumors are defined as histologically confirmed metastatic malignancies, and the primary region is not recognized before treatment [1]. Occult thyroid carcinoma is common in the clinic; however, occult anaplastic thyroid cancer (ATC) is rare [2,3]. As far as our knowledge, there is none of reported case of occult ATC presenting as a renal metastatic lesion because almost all ATC patients present with a rapidly growing neck mass. However, distant metastases are found in early disease presentations in about half of patients. In particular, some have metastases to the kidneys [4]. Herein, we report the first case of occult anaplastic thyroid cancer which was initially considered to be a metastatic renal cell carcinoma (RCC).
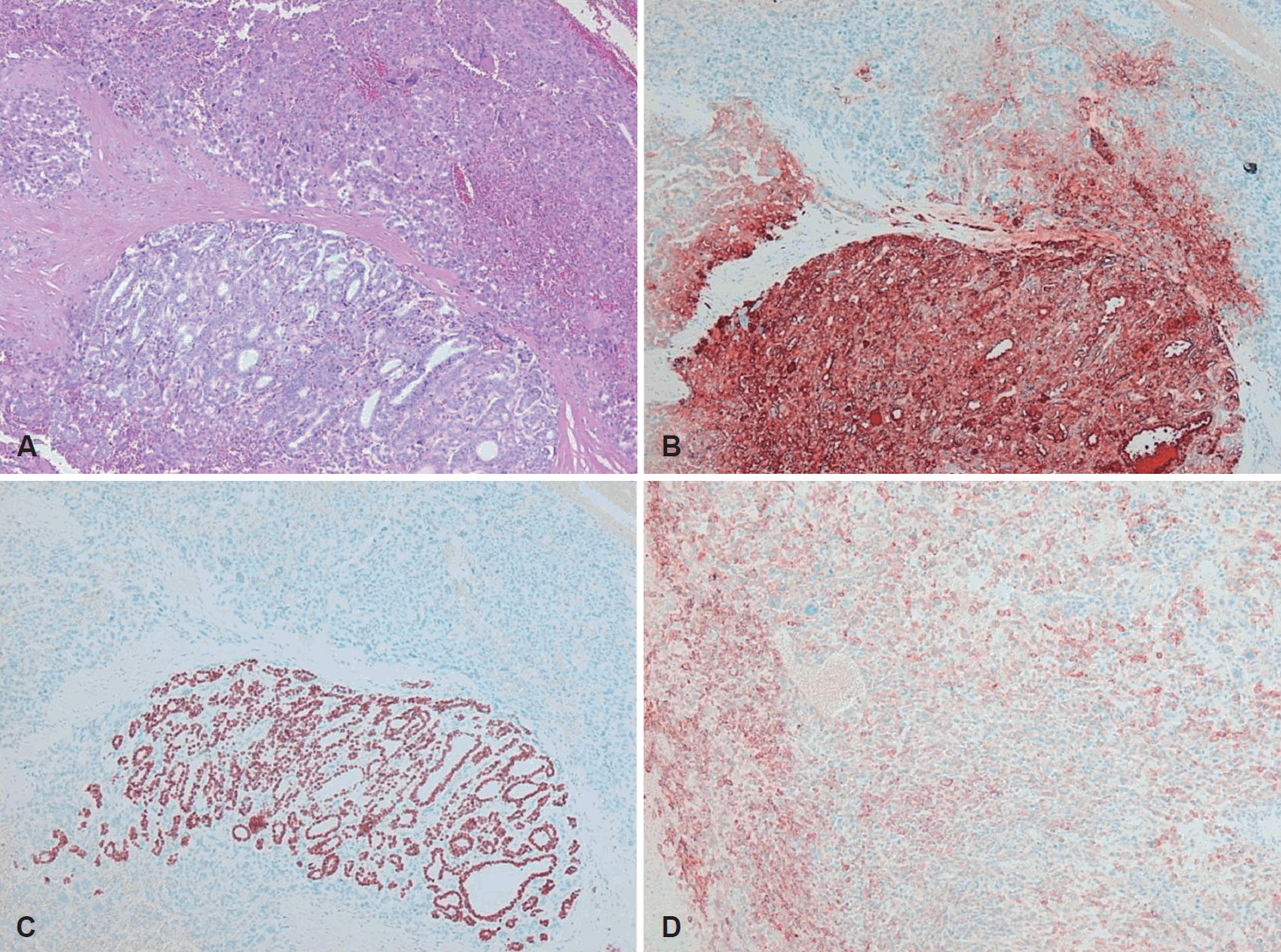
A 68-year-old patient was referred to the department of urology at our institution with a huge left renal mass which was detected incidentally on ultrasonography during the evaluation of abdominal pain. She had suffered from abdominal pain for 2 months before presentation. Physical examination presented a palpable mass in the left upper quadrant of the abdomen. No abnormal values were found in her blood chemistry with the exception of microscopic hematuria (26-30/HPF) on urine analysis. Computed tomography (CT) showed a 12-cm exophytic cystic tumor in the left kidney, a 3.2-centimeter infiltrative mass in the paraumbilical portion of the liver, and small nodules in both basal lung fields (Fig. 1). Bone scan did not find any evidence of bone metastasis. Positron emission tomography (PET)-CT presented hypermetabolic nodules in the right thyroid gland, both lungs, liver, left kidney and left paraaortic and common iliac lymph nodes (Fig. 2A and B). These findings were suggestive of metastatic RCC. Sono-guided percutaneous needle biopsy of the hepatic mass was done, but no cancer component was revealed from the biopsy. In addition, the biopsy result was indicative of thyroid tissue showing a follicular structure containing colloid-like material, which was positive in immunohistochemistry stain with thyroglobulin (TG) and thyroid transcription factor-1 (TTF-1). Although our pathologist recommended a repeat biopsy, we considered that the mass could be a kind of RCC. We subsequently performed left total nephrectomy not only for the palliation of uncontrolled severe abdominal pain but also with the aim to administer adjuvant therapy. In addition, cytoreductive nephrectomy for metastatic RCC is known to improve median survival [5]. However, the final histopathologic result demonstrated that the renal mass was from ATC (Fig. 3). Microscopically, it presented a papillary and follicular proliferation with colloid like material in the intra-follicular space. The strongly positive immunohistochemical staining with TG and TTF-1 verified the origin from the thyroid gland and the diagnosis of ATC metastasising to kidney. Consequently, thyroid ultrasonography and fine needle aspiration cytology was done. Sub-centimeter sized ill-defined hypoechoic nodules with calcification were noted on right side of the thyroid gland diagnosed as nodular hyperplasia of the thyroid (Fig. 2C and D). After the operation, the patient was supposed to receive systemic chemotherapy or target therapy according to the biopsy result, but only supportive care was given due to her poor general condition and refusal of treatment. The patient showed an aggressive course despite appropriate supportive treatment. In spite of every effort of supportive care, the patient developed pulmonary thromboembolism with severe airway compromise due to multiple hematogenous lung metastasis and expired from respiratory distress 8 weeks after the surgery.
The initial symptom of ATC is an extremely rapidly growing neck mass, occurring in about 85% of patients with ATC. The ATC may cause tenderness and neck pain, and compression (or direct invasion) of the airway and digestive organs, causing dyspnea (about 35% of patients), swallowing difficulty (30%), hoarseness (25%), and cough (and sometimes hemoptysis, 25%). Less common clinical manifestation are chest discomfort, headache, bone pain, mental confusion, or abdominal pain from metastases [6].
Distant metastases are recognized at initial evaluation in 15% to 50% of patients [4,7-9]. ATC usually metastasizes to distant organs via the bloodstream. The lung is the most common site of metastasis, up to 90% of patients with distant disease are involved [8,9]. The second most common regions of distant disease are the bone and brain, and some patients have metastatic legions to the skin, kidneys, liver, pancreas, heart and adrenal glands [8-11]. Metastasis to the kidney caused by ATC is found in 16% of patients at postmortem autopsy [12]. However, only one case has been reported with renal metastasis of ATC [8].
Occult ATC is very rare. Aldinger, et al. [13] reported ATC in a patient with neck free from disease. In this case, papillary-follicular carcinoma was found in the thyroid gland. After 8 years, metastatic ATC was present at postmortem examination. Takashima, et al. [3] reported that ATC with metastatic lymph node mimicked a parathyroid tumor.
ATCs are severely aggressive disease, with a disease-specific mortality approaching 100%. In a review of published case series (1771 patients treated between 1949 and 2007), the median survival was 5 months, and the 1-year survival was 20% [14]. The median survival in patients with ATC with distant metastases at the time of diagnosis was 4.2 months, compared with 6 months in those without metastases [15]. In patients with advanced tumor, palliation of symptoms is a high priority.
In our patient, the authors considered the disease to be an advanced RCC with multiple metastases at presentation. Initially, we had planned neoadjuvant targeted therapy because the patient had good performance status at that time. Subsequently, we performed sono-guided percutaneous needle biopsy of the hepatic lesion to clarify the cell type of the RCC. However, unfortunately no cancer component was revealed by the biopsy, and, in addition, the biopsy result was indicative of thyroid tissue showing a follicular structure containing colloid-like material, which was positive in immunohistochemical staining with TTF-1 and TG. Although our pathologist recommended repeat biopsy, the authors still considered that the mass could be a kind of RCC at that time. In addition, cytoreductive nephrectomy was inevitabllly decided for palliative treatment due to severe uncontrolled abdominal pain. This case shows that repeated biopsies should be carried out before cytoreductive nephrectomy for proper management whenever the preoperative biopsy result is not appropriate to confirm a renal cancer. Furthermore, we should remember that ATC can metastasize to the kidney, and the neck may be free from disease.
This study was reviewed and approved by Institutional Review Board: CUH 2020-07-010.
REFERENCES
1. Greco FA, Hainsworth JD. Cancer of unknown primary site. In : DeVita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology. 8th ed. Philadelphia: Lippincott Williams & Wilkins;2008. p. 2363–87.
2. Deutschmann M, Khalil M, Bhayana S, Chandarana S. Occult multifocal papillary thyroid microcarcinoma presenting as a supraclavicular mass containing anaplastic thyroid carcinoma. JAMA Otolaryngol Head Neck Surg. 2013; 139(4):415–8.

3. Takashima S, Morimoto S, Ikezoe J, Kokado Y, Kozuka T. Occult anaplastic thyroid carcinoma associated with marked hypercalcemia. J Clin Ultrasound. 1990; 18(5):438–41.

4. McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, et al. Anaplastic thyroid carcinoma: A 50-year experience at a single institution. Surgery. 2001; 130(6):1028–34.

5. Conti SL, Thomas IC, Hagedorn JC, Chung BI, Chertow GM, Wagner TH, et al. Utilization of cytoreductive nephrectomy and patient survival in the targeted therapy era. Int J Cancer. 2014; 134(9):2245–52.

6. Lip GY, Jaap AJ, McCruden DC. A presentation of anaplastic carcinoma of the thyroid with symptomatic intra-abdominal metastases. Br J Clin Pract. 1992; 46(2):143–4.
7. Nel CJ, van Heerden JA, Goellner JR, Gharib H, McConahey WM, Taylor WF, et al. Anaplastic carcinoma of the thyroid: A clinicopathologic study of 82 cases. Mayo Clin Proc. 1985; 60(1):51–8.

8. Carcangiu ML, Steeper T, Zampi G, Rosai J. Anaplastic thyroid carcinoma. A study of 70 cases. Am J Clin Pathol. 1985; 83(2):135–58.

9. Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990; 66(2):321–30.

10. Tan RK, Finley RK 3rd, Driscoll D, Bakamjian V, Hicks WL Jr, Shedd DP. Anaplastic carcinoma of the thyroid: A 24-year experience. Head Neck. 1995; 17(1):41–7. discussion 47-8.

11. Hadar T, Mor C, Shvero J, Levy R, Segal K. Anaplastic carcinoma of the thyroid. Eur J Surg Oncol. 1993; 19(6):511–6.
12. Heitz P, Moser H, Staub JJ. Thyroid cancer: A study of 573 thyroid tumors and 161 autopsy cases observed over a thirty-year period. Cancer. 1976; 37(5):2329–37.
13. Aldinger KA, Samaan NA, Ibanez M, Hill CS Jr. Anaplastic carcinoma of the thyroid: A review of 84 cases of spindle and giant cell carcinoma of the thyroid. Cancer. 1978; 41(6):2267–75.
Fig. 1.
Contrast-enhanced CT images showing a 12-cm exophytic cystic mass in the left kidney with intensely enhanced solid portion (A), a 3.2-cm infiltrative mass in the paraumbilical portion of the liver (arrow) (B), and small enhanced nodules in both basal lung fields (arrow) (C). PET-CT revealed hypermetabolic nodules in the right thyroid gland, both lungs, liver, left kidney and left paraaortic and common iliac lymph nodes (D).
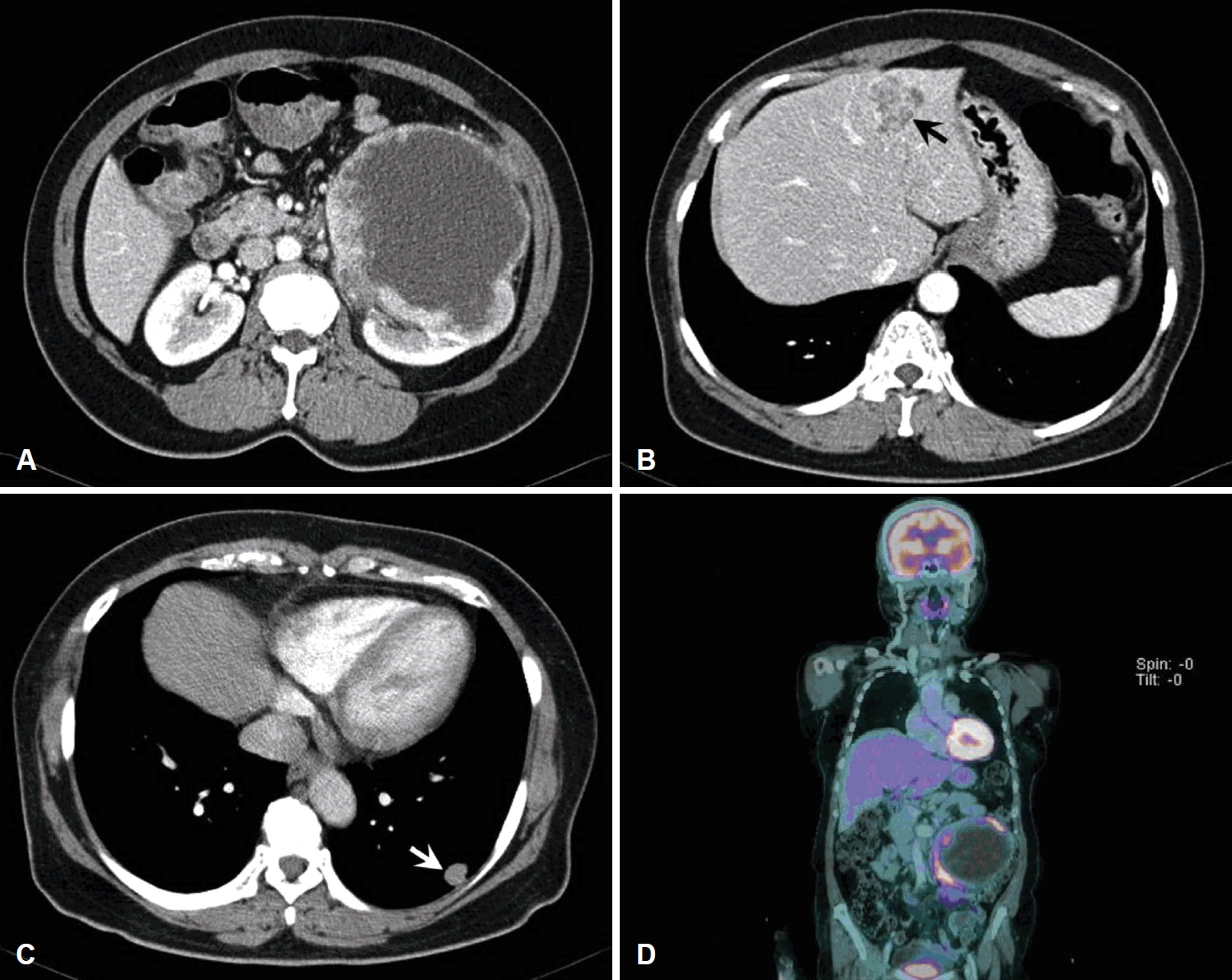
Fig. 2.
Examinations to confirm thyroid origin. Contrast-enhanced CT images showing thyroid nodules with microcalcification in the right thyroid gland (A). PET-CT revealed hypermetabolic nodules in the right thyroid gland (B). Thyroid ultrasonography showing (C) 0.633 cm sized ill-defined hypoechoic nodule with calcification noted on right thyroid gland diagnosed as nodular hyperplasia on fine needle aspiration cytology and (D) 0.477 cm sized well-defined mixed echoic nodule with calcification noted on right thyroid gland.
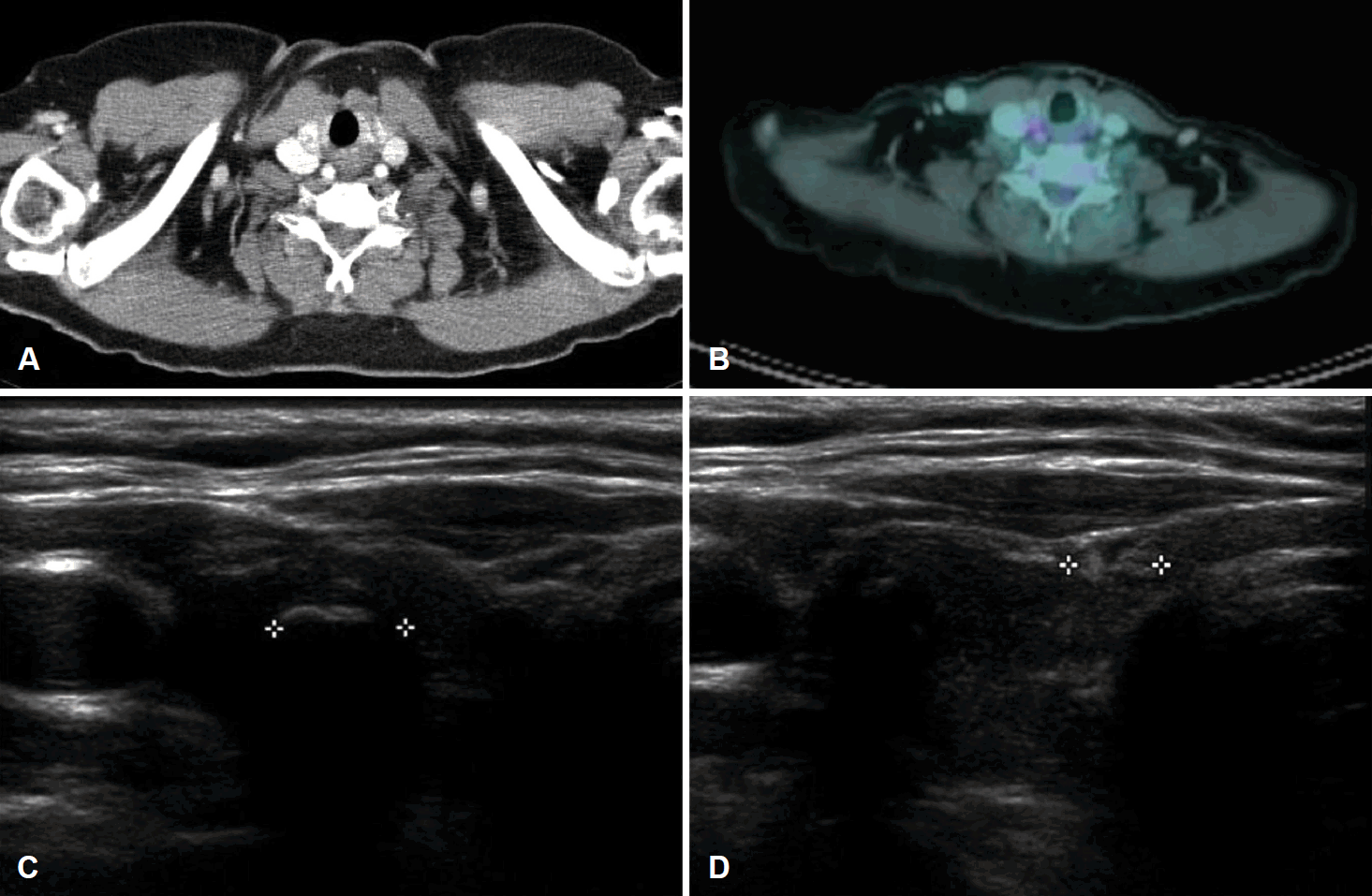
Fig. 3.
Histopathological findings. Round-to-oval shaped follicle lined with follicular cells, however, colloidal material not seen (lower part of image), and marked degree of pleomorphism pattern (upper part of image) (hematoxylin and eosin, ×100) (A). Strong cytoplasmic expression of TG on round-to-oval shaped follicle (lower part of image) (TG, ×100) (B). Strong nuclear expression of TTF-1 on round-to-oval shaped follicle (lower part of image) (TTF-1, ×100) (C). Moderate cytoplasmic expression of epithelial membrane antigen on tumor cells (epithelial membrane antigen, ×100) (D). TG: Thyroglobulin, TTF-1: thyroid transcription factor-1.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download