Abstract
Sebaceomas of the external auditory canal (EAC) are extremely rare. The recognition of sebaceoma is important as this benign tumor can be be part of the spectrum of the Muir-Torre syndrome (MTS), which is associated with visceral malignancy and multiple adenomatous polyps. Proper histological identification is thus important for further investigation. We describe a rare case of a sebaceoma that was confined to the EAC. Subsequent immunohistochemical (IHC) staining analysis of the mismatch repair genes (MLH1, MSH2, MSH6, and PMS2) was needed to investigate MTS. Even though the patient has had the history of colon cancer, the result was negative. Otorhinolaryngologists should be aware of rare sebaceoma and its potential association with the internal malignancy, besides the limitation of IHC screening test.
Sebaceoma is a benign tumor that usually presents as a solitary papule or nodule on the scalp [1]. This tumor can occur in any skin containing hair and sebaceous glands. Sebaceoma of the external auditory canal (EAC) is extremely rare [1,2]. This neoplasm can be part of the spectrum of the Muir-Torre syndrome (MTS), in which it may be accompanied by visceral neoplasms, most commonly gastrointestinal. We report a rare case of a sebaceoma at EAC with history of colorectal cancer at 5 years ago. He underwent immunohistochemical (IHC) staining analysis of the mismatch repair (MMR) genes (MLH1, MSH2, MSH6, and PMS2) and did not reveal any mutation, in spite of previous colorectal cancer history.
A 49-year-old man with a slow growing mass in the left EAC visited our outpatient department. He had a surgical history of adenocarcinoma at rectosigmoid colon 5 years ago.
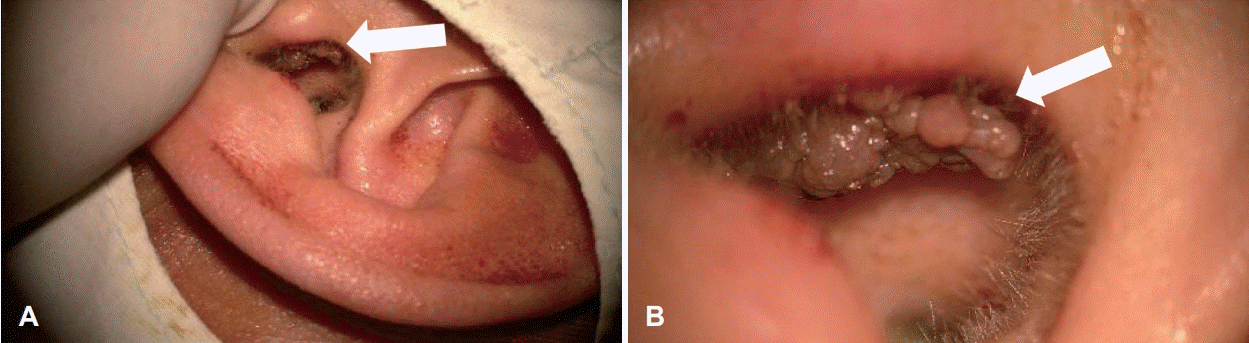
Physical examination revealed an 1 cm sized firm, cauliflower looking exophytic mass in the posterior side of the tragus, centered at the helical root, without skin ulceration (Fig. 1). The remainder of the physical examination was unremarkable. The patient underwent wide local excision under the microscope. Closure of the tragal skin defect was accomplished by split-thickening skin graft. The post-operative recovery was uneventful, and there was no recurrence during 6 months of follow-up.
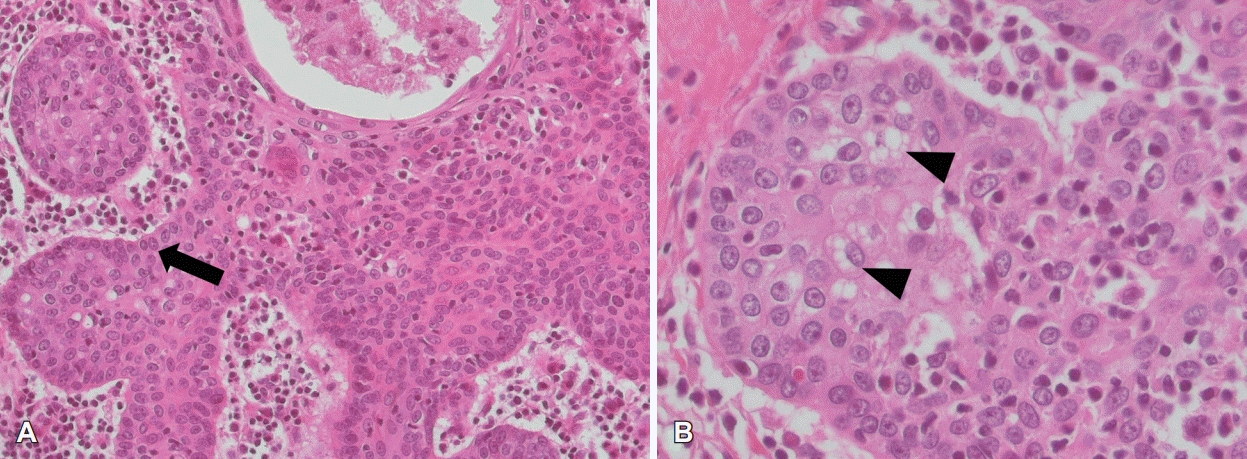
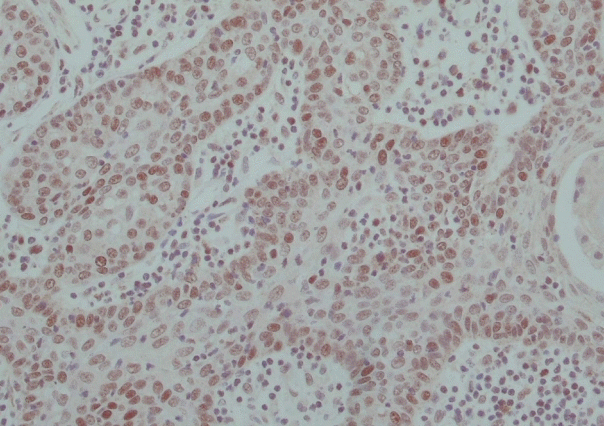
Histopathological examination revealed a well-demarcated nodule comprised irregularly shaped masses of basaloid cell, consistent with a sebaceoma (Fig. 2). Due to newly diagnosed sebaceoma, he was recommended to do further investigation of additional malignancy and MTS. The IHC staining to detect MLH1, MSH2, MSH6, and PMS2 mutation was done, but all of them turned out to be negative (Fig. 3).
Sebaceoma is a benign non melanocytic neoplasm, first coined by Troy in 1984 [1]. Sebaceoma has been found at the nose, face, scalp, and neck [2]. The skin of the auricle and EAC contains several types of adnexal secretory glands such as eccrine, apocrine, and sebaceous glands. Differential diagnosis in EAC mass include benign lesions of epidermis, such as wart, papilloma, seborrheic keratosis, atheroma and granuloma fissuratum, as well as basal cell carcinoma and squamous cell carcinoma.
Clinically, it presents as a flesh colored, asymptomatic, papulonodule that occurs almost exclusively on the face, particularly nose and cheek [1].
Sebaceoma is defined as a benign adnexal tumor with microscopic characteristics of minor population of clear cells with multivacuolated cytoplasm, consistent with mature sebaceous cells (nuclear hyperchromasia with indentations), that are not identified by any known types of IHC staining [3]. Sebaceoma can be differentiated histologically from other tumors which may display sebaceous differentiation, such as trichoblastoma, apocrine poroma and nodular basal cell carcinoma [2].
MTS is a rare autosomal dominant cancer susceptible condition considered to be a phenotypic variant of the Lynch syndrome (hereditary non-polyposis colorectal cancer) [4]. The skin manifestation of MTS may be concurrent (22%), antecedent (6%), or subsequent (56%) to the diagnosis of visceral cancer [5,6]. The diagnostic criteria consist of one or multiple sebaceous neoplasm (sebaceous adenomas, carcinomas, or epithelioma) in association with colorectal or genitourinary cancers [7,8]. Because of that, the identification of MTS has important implications for the patient and their family [9]. IHC staining for MLH1, MSH2, MSH6, and PMS2 can confirm the diagnosis [4,7-11].
Our patient has a history of a colon adenocarcinoma at the age of 44 years treated with low anterior resection. At the time of his sebaceoma diagnosis, possibility of MTS should be investigated. Besides, the patient should undergo a complete work up with thorough physical examinations, colonoscopies, appropriate imaging and prompt assessment for MMR gene mutation [12]. IHC staining result of this case turned out to be negative.
However, it is also important to be well-informed of under the limitations of IHC screening testing. Of course, sebaceous lesions on the trunk or extremities, compared with the head or neck were significantly more likely to exhibit MMR deficiency (64.7% vs. 25.2%; p<0.01) [10,13]. These findings were suggesting that the increased ultraviolet exposure to head and neck contributes to the development of MMR-proficient sebaceous neoplasia [14]. Mutation in MMR deficiency is detected in just 66% of sebaceoma [15]. Furthermore, the abnormal IHC had an 85% sensitivity, 48% specificity, 22% positive predictive value and 95% negative predictive value when evaluating for MTS [10]. As a whole, MMR-deficiency is observed in 30-45% of sebaceous lesions. Nevertheless, antibodies against MMR proteins should be used as a first step in ruling out the MTS. Given the current test results, it would be more reasonable that this case was not associated with MTS.
In conclusion, otorhinolaryngologists should be aware of the possible occurrence of sebaceoma in the EAC. Furthermore, we should know patients with sebaceoma have potential association with the MTS including internal malignancy. Furthermore, the all first-degree relatives of MTS patient, especially mutation carriers, should be referred for skin exams, colonoscopies, and gynecologic screening including transvaginal ultrasound exams. Initial evaluation should be started as early as possible.
Notes
Author Contribution
Conceptualization: Jeong Hwan Choi. Data curation: Jeong Hwan Choi, Young Nam Kim. Formal analysis: Young Nam Kim, Chan Young Lee. Investigation: Jeong Hwan Choi, Young Nam Kim. Methodology: Jeong Hwan Choi. Project administration: Jeong Hwan Choi. Resources: Jeong Hwan Choi, Chan Young Lee. Software: Young Nam Kim. Supervision: Jeong Hwan Choi. Validation: Jeong Hwan Choi, Kyeong Mee Park. Visualization: Kyeong Mee Park, Young Nam Kim. Writing—original draft: Young Nam Kim. Writing—review & editing: Jeong Hwan Choi, Kyeong Mee Park.
REFERENCES
1. Magliulo G, Colicchio MG, Ciniglio Appiani M, Minni A, Marcotullio D. Sebaceoma of the external auditory canal. Otol Neurotol. 2010; 31(7):1175–6.

2. Jacobson JP, Weisstuch A, Hajdu C, Myssiorek D. Sebaceoma of the auricle. J Laryngol Otol. 2012; 126(8):830–2.

3. Cassarino DS. Diagnostic pathology: Neoplastic dermatopathology. Philadelphia: Lippincott Williams & Wilkins, Amirsys;2012.
4. Hare HH, Mahendraker N, Sarwate S, Tangella K. Muir-Torre syndrome: A rare but important disorder. Cutis. 2008; 82(4):252–6.
5. Cesinaro AM, Ubiali A, Sighinolfi P, Trentini GP, Gentili F, Facchetti F. Mismatch repair proteins expression and microsatellite instability in skin lesions with sebaceous differentiation: A study in different clinical subgroups with and without extracutaneous cancer. Am J Dermatopathol. 2007; 29(4):351–8.

6. Kruse R, Ruzicka T. DNA mismatch repair and the significance of a sebaceous skin tumor for visceral cancer prevention. Trends Mol Med. 2004; 10(3):136–41.

7. Baiocchi GL, Chiocchi E, Vermi W, Molfino S, Gheza F, Biasca F, et al. The Muir-Torre syndrome: A typical case of misdiagnosis and consequent worsened prognosis. Int J Colorectal Dis. 2015; 30(3):431–2.

8. Ponti G, Manfredini M, Tomasi A, Pellacani G. Muir-Torre syndrome and founder mismatch repair gene mutations: A long gone historical genetic challenge. Gene. 2016; 589(2):127–32.

9. Schon K, Rytina E, Drummond J, Simmonds J, Abbs S, Sandford R, et al. Evaluation of universal immunohistochemical screening of sebaceous neoplasms in a service setting. Clin Exp Dermatol. 2018; 43(4):410–5.

10. Roberts ME, Riegert-Johnson DL, Thomas BC, Thomas CS, Heckman MG, Krishna M, et al. Screening for Muir-Torre syndrome using mismatch repair protein immunohistochemistry of sebaceous neoplasms. J Genet Couns. 2013; 22(3):393–405.

11. Bogani G, Leone Roberti Maggiore U, Raspagliesi F. Lynch syndrome - Muir-Torre variant: Implication in gynecologic oncology. J Gynecol Oncol. 2018; 29(5):e84.

12. Kennedy RA, Thavaraj S, Diaz-Cano S. An overview of autosomal dominant tumour syndromes with prominent features in the oral and maxillofacial region. Head Neck Pathol. 2017; 11(3):364–76.

13. Walsh MD, Jayasekara H, Huang A, Winship IM, Buchanan DD. Clinico-pathological predictors of mismatch repair deficiency in sebaceous neoplasia: A large case series from a single Australian private pathology service. Australas J Dermatol. 2019; 60(2):126–33.

Fig. 1.
Preoperative oto-endoscopic views of the external auditory canal. Otoendoscopic examination reveals a firm, about 1 cm, cauliflower-like mass (arrow) in the concha (A). In magnification view, the mass (arrow) has rough surface without skin ulceration (B).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download