Abstract
Primary ciliary dyskinesia (PCD) results in several characteristic clinical symptoms, including chronic pansinusitis, recurrent infections of the respiratory tract, and infertility. Concerning the rhinologic aspect, medical therapy mainly involving a combination of long-term antimicrobial agents and intranasal steroid sprays can control sinusitis in the majority of cases. But, there are no definite treatment guidelines for recalcitrant chronic sinusitis with PCD. Recently, we examined a 28-year-old male with serous otitis effusion, chronic sinusitis, and bronchiectasis, and a 4-year-old female with chronic sinusitis and serous otitis effusion. We confirmed PCD by electron microscopy and endoscopic maxillary mega-antrostomy was performed in both cases for the treatment of chronic sinusitis that was refractory to conservative management.
Primary ciliary dyskinesia (PCD) is an autosomal recessive disorder leading to the impairment of ciliary function and mucosal clearance leading to recurrent upper and lower respiratory tract infections. Since the first report by Afzelius [1] on the abnormalities observed in the microtubule structure and arrangement of the cilia by electron microscopy, Min, et al. [2] reported 19 cases of PCD in Korea. Previous studies were focused on diagnosis and medical treatment of PCD, and there are no definite surgical guidelines for chronic sinusitis treatment with PCD. We recently examined a 28-year-old male and 6-year-old female with PCD. Both were treated successfully with endoscopic maxillary mega-antrostomy (EMMA). These cases may be suggestive of an alternative surgical treatment. This study was exempted from deliberation by the Ethics Committee of the Konkuk University Hospital.
A 28-year-old male presenting with a persistent nasal obstruction and hearing impairment visited the outpatient clinic of our rhinology department. After a previous diagnosis of asthma at a different hospital, the patient was found to have mucopurulent discharge in both nasal cavities making breathing through the nasal passage impossible. Serous otitis media was observed in the right middle ear. High resolution chest computed tomography (CT) showed the presence of bronchiectasis. We suspected PCD, and performed the saccharine test, which revealed delayed ciliary movement over a 60-minute period. For the confirmation of PCD, biopsy at the left inferior turbinate mucosa was conducted. Electron microscopy demonstrated that the dynein arms were decreased (Fig. 1). Para nasal sinus (PNS) CT showed both pansinusitis with mucosal thickening and inflammatory change. Bilateral endoscopic sinus surgery (ESS) was performed under local anesthesia. In the surgical field, a diffuse-inflammatory mucosal change was observed in both nasal cavities and the patient received bilateral EMMA. The surgical technique was performed as follows. The maxillary antrostomy was enlarged posteriorly with through-cutting forceps to the level of posterior wall of the maxillary sinus. Next, to allow inferior enlargement of the antrostomy, the posterior half of the inferior turbinate was resected using back-biting punch forceps and a microdebrider. Down-biting punch forceps were used to resect the medial wall of the maxillary sinus from the antrostomy to the nasal floor. As a result, the maxillary antrostomy was maximally enlarged posteriorly and inferiorly [3]. For simultaneous treatment of bronchiectasis, oral prednisolone (5 mg) was maintained if the condition worsened. During a 1-year follow-up in the outpatient clinic, no recurrence was noted (Fig. 2A and B).
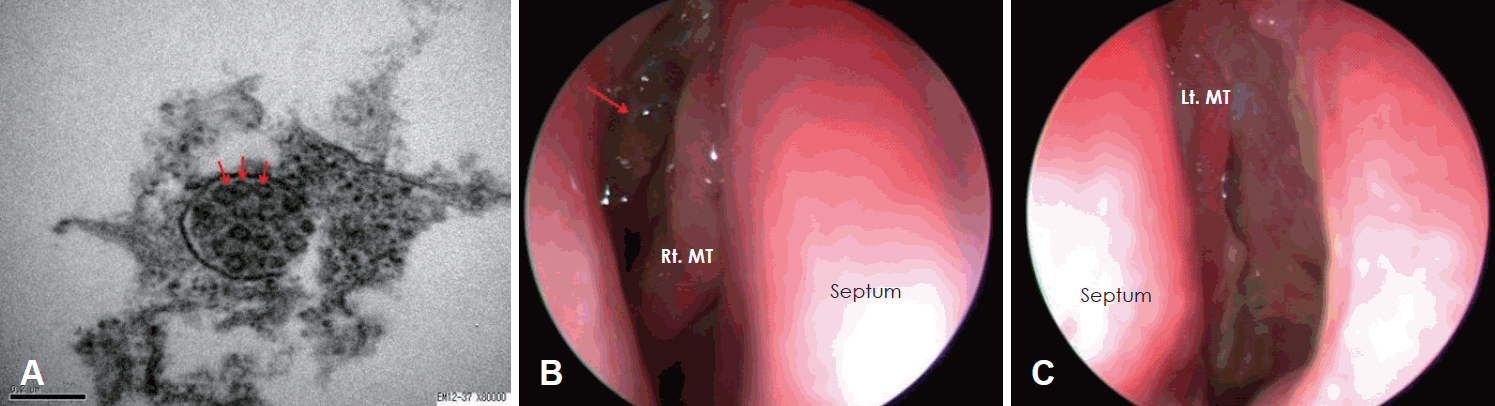
A 6-year-old female presenting with a persistent nasal obstruction and hearing impairment visited outpatient clinic of rhinology department. With the diagnosis of chronic sinusitis and serous otitis effusion, she had undergone bilateral ESS and ventilation tube insertion in another hospital 3 years previously. Upon physical examination, the patient was found to have diffuse nasal polyps in both nasal cavities and serous otitis media in both middle ears. A chest roentgenogram demonstrated a normal appearance. The serum blood test exhibited prolonged activated partial thromboplastin time (aPTT= 48.9). In screening serum blood test for prolonged aPTT, serum antinuclear antibody level was verified by a 2+, speckled pattern. The pediatrics department assumed any form of autoimmune disease. Concerning rhinology, we suspected nasal mucosal lesion and performed PNS CT. The examination demonstrated pansinusitis and the saccharine test revealed delayed ciliary movement over a 60-minute period. PCD was confirmed upon biopsy at the left inferior turbinate mucosa and electron microscopy demonstrated that the microtubules were irregularly paired (Fig. 3). We performed bilateral EMMA for the treatment of PCD. However, the posterior part of inferior turbinate was minimally resected to consider the growth of nasal cavity. For postoperative management, the patient underwent saline irrigation with budesonide intranasal spray. During a 9-months follow-up in the outpatient clinic, she exhibited no clinical symptoms, except a minimal mucosal polypoid lesion.
Originally, a study by Eliasson and Bisgaard [4] identified abnormal mucociliary transport due to microtubular defects of the cilia as immotile cilia syndrome. Rossman and Newhouse [5] revised the definition to dyskinetic cilia syndrome because the cilia were not completely immotile, and the clinical features were introduced in several reports. PCD has been misdiagnosed as chronic upper and lower respiratory tractinfections; misdiagnosis or delayed diagnosis of PCD has caused the initiation of more severe symptoms including bronchiectasis and pulmonary interstitial fibrosis resulting in fluid-collection with recurrent pneumonia [4]. Therefore, an expedited and exact diagnosis is a key element for the proper treatment and clinical pathway control of PCD.
Suspected PCD requires clarification through evaluation of clinical features, electron microscopic examination of the ciliary ultrastructure, study of mucociliary transport, and analysis of ciliary movement [5]. The evaluation of clinical features involves the clinical history including familiar history, imaging studies including CT, and a pulmonary function test [6]. The saccharin test, used as a screening test to evaluate the mucociliary clearance is relatively inexpensive, simple, repeatable, and convenient to apply in the clinical setting. However, the test is only available to check the flow rate, and can be subjective depending on the saccharin arrival to the gustatory bud [7]. In this report, we conducted the saccharin test before surgery, with a measured time of over 60 minutes that gave the clue for the suspected PCD diagnosis. However, the delayed time was not always constant, so the alternative test would be necessary.
Through the use of tannic acid within the fixation solution, Mizuhira and Amakawa [8] resolved the issue regarding the limited detection of the inner dynein arm in cross sectional images with electron microscopy, and it is now possible to investigate all of the dynein arms. The most common ciliary ultrastructural abnormality is the congenital defect of the dynein arm. This observation is also supported by our presentation that demonstrated a partial defect of the inner and outer dynein arm with electron microscopy in previous case (type Id) [9]. But, we also found microtubular abnormality in the latter case that demonstrated irregular pairing (type III).
The main purpose of chronic sinusitis treatment with PCD is the improvement of the mucociliary transport facility, ESS is beneficial to fluid-drainage and relieving symptoms from the chronic sinusitis [10], and the inferior meatal antrostomy (IMA) was recommended for chronic maxillary sinusitis with PCD [11]. But, based on Lund’s study [12], we did not assure that the IMA would be more beneficial than middle meatal antrostomy (MMA). Thus, the MMA did not seem to be sufficient to the intractable chronic sinusitis. EMMA was introduced as a mucosal sparing technique that facilitates mucus clearance and sinus irrigation in terminally dysfunctional maxillary sinuses. EMMA involves resection of the posterior half of the inferior turbinate and extending the antrostomy down to the floor of the nose, creating a significantly enlarged antrostomy. However, EMMA was so effective for treatment of recalcitrant chronic sinusitis with previous Caldwell-Luc operation and cystic fibrosis which decreased mucociliary function that we performed this alternative surgical technique [3]. There was no report that showed successful results using EMMA as treatment for PCD induced chronic maxillary sinusitis, as we reported for these cases. There are three main reasons for using EMMA to treat PCD. First, remaining mucociliary clearance is preserved. Secondly, the approach is novel rather than a traditionally utilization of IMA, which easily results in re-stenosis. Thirdly, nasal irrigation during postoperative following is easily accomplished. Other advantages of EMMA are the preservation of the function of humidification, filtration and heating of inspired air, and the theoretical complications of EMMA included atrophic rhinitis, although this is not observed in other reports, nasolacrimal duct injury, and primarily bleeding [3]. In PCD patient, specific surgery technique has not been reported for ethmoid sinus, frontal sinus and sphenoid sinus. There is a limitation that EMMA could only be used in the maxillary sinus in that cases.
In conclusion, it is difficult to detect and treat PCD related chronic sinusitis from the aspect of rhinology. Based on two cases of chronic sinusitis treated by surgical approach, we carefully suggest that EMMA could be an alternative surgical treatment for recalcitrant chronic sinusitis with PCD.
Notes
Author Contribution
Conceptualization: all authors. Formal analysis: Jin Kook Kim, Joon Yong Park, Taesik Jung. Funding acquisition: all authors. Project administration: Jin Kook Kim. Supervision: all authors. Validation: all authors. Writing—original draft: Haemin Noh. Writing— review & editing: Jin Kook Kim.
REFERENCES
2. Min YG, Shin JS, Choi SH, Chi JG, Yoon CJ. Primary ciliary dyskinesia: Ultrastructural defects and clinical features. Rhinology. 1995; 33(4):189–93.
3. Cho DY, Hwang PH. Results of endoscopic maxillary megaantrostomy in recalcitrant maxillary sinusitis. Am J Rhinol. 2008; 22(6):658–62.

4. Ellerman A, Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. Eur Respir J. 1997; 10(10):2376–9.

5. Rossman CM, Newhouse MT. Primary ciliary dyskinesia: Evaluation and management. Pediatr Pulmonol. 1988; 5(1):36–50.

6. Schidlow DV. Primary ciliary dyskinesia (the immotile cilia syndrome). Ann Allergy. 1994; 73(6):457–68. quiz 468-70.
7. Andersen I, Camner P, Jensen PL, Philipson K, Proctor DF. Nasal clearance in monozygotic twins. Am Rev Respir Dis. 1974; 110(3):301–5.
8. Mizuhira B, Amakawa T. [Electron microscopic studies on the histocytochemistry and the physiological function of the kidney]. Nihon Rinsho. 1972; 30(6):1307–21.
9. Herzon FS, Murphy S. Normal ciliary ultrastructure in children with Kartagener̕s syndrome. Ann Otol Rhinol Laryngol. 1980; 89(1 Pt 1):81–3.

10. Parsons DS, Greene BA. A treatment for primary ciliary dyskinesia: Efficacy of functional endoscopic sinus surgery. Laryngoscope. 1993; 103(11 Pt 1):1269–72.
11. Rha KS. Diagnosis and treatment of sinusitis in children. J Clinical Otolaryngol. 1999; 10(2):147–56.

12. Lund VJ. Fundamental considerations of the design and function of intranasal antrostomies. Rhinology. 1985; 23(3):231–6.
Fig. 1.
In electron microscopy images, a reduction in the number of outer dynein arms (arrow) and inner dynein arms (arrowhead) was evident.
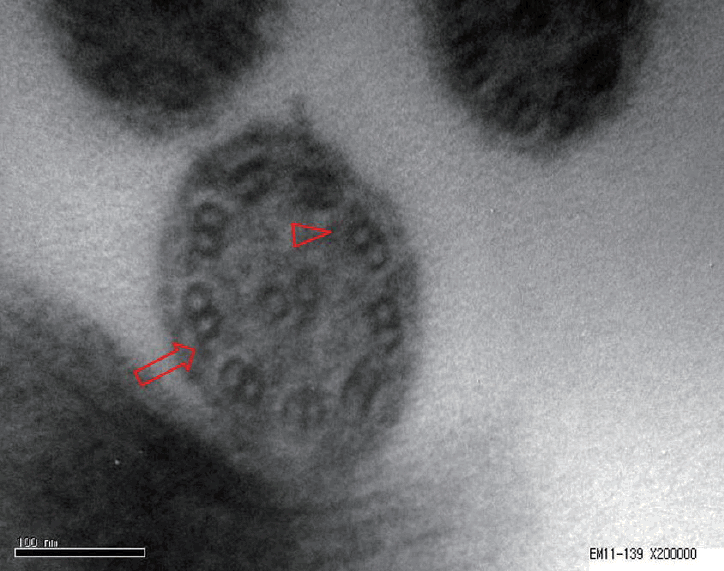
Fig. 2.
In postoperative endoscopic finding, right side (A) and left side (C) nasal cavity demonstrated well healed mucosal lesion and clearance. The mega-antrostomy site (B) also demonstrated mucosal clearance twelve months after the operation. Rt. MT: right middle turbinate, Lt. MT: left middle turbinate.
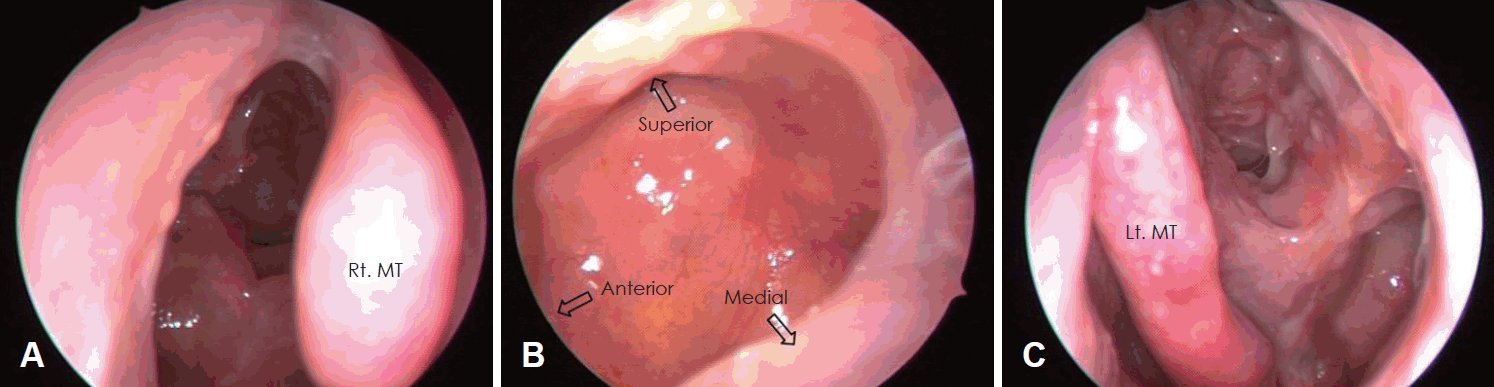
Fig. 3.
In electron microscopy imaging, the axoneme of the cilium consisted of irregular pairs of microtubules (arrow), demonstrated type III (A). The postoperative telescopic findings of second case showed no recurrence except minimal mucosal polypoid lesion (arrow, right side-B; left side-C). Rt. MT: right middle turbinate, Lt. MT: left middle turbinate.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download