Abstract
Background and Objectives
Snoring is the most common symptom of obstructive sleep apnea (OSA) and is caused by turbulent airflow due to narrowing of the upper airways. In patients with positional OSA, a change in sleep posture from supine to lateral is known to reduce snoring and sleep apnea. This study was performed to compare changes in snoring sound intensity and formant frequencies according to sleep position.
Subjects and Method
A total of 19 patients (male: 18; female: 1) diagnosed with positional OSA by polysomnography (PSG) were enrolled in this study. The snoring sounds recorded during PSG were analyzed acoustically and compared according to sleep position (i.e., supine vs. lateral).
Results
Snoring disappeared on changing sleep position in five patients, all of whom had Apnea-Hypopnea Index (AHI) <15. In other patients, the snoring sounds tended to decrease with posture change, and the degree of decrease was inversely proportional to AHI (p=0.015) and respiratory disturbance index (RDI) (p=0.013). Formant frequencies 1, 3, and 4 (F1, F3, and F4, respectively) decreased when sleeping in the lateral position (p=0.02, 0.03, and 0.01, respectively).
Sleep disordered breathing (SDB), a chronic disease in which breathing partially or completely ceases during sleep, has a worldwide prevalence of 4-7%. Obstructive sleep apnea (OSA), the most common form of SDB, is characterized by symptomatic and repetitive partial or complete collapse of the airway [1,2]. This instability arises from the structural vulnerability of the upper airway and loss of muscle tone during sleep [3]. Recently, OSA has been recognized as an important public health problem, being associated with an increased risk of cancer, atherosclerosis, cardiovascular disease, stroke, hypertension, and mental health problems such as dementia and cognitive abnormalities [4-9]. In OSA patients, sleep position affects the frequency of obstructive respiratory events [10]. In the supine position, OSA is exacerbated in terms of apnea frequency, duration, and desaturation, as well as the duration of arousals. Positional dependency is defined as a supine apnea-hypopnea index (AHI) at least twice that of a nonsupine AHI, and is evident in 50-70% of OSA patients [3].
Snoring, the most common symptom of OSA, is caused by turbulent airflow through narrowed upper airways [1]. Nakano, et al. [11] showed that snoring intensity and duration decreased when OSA patients changed their sleep posture from supine to lateral. However, this was observed only in patients with AHIs less than 15. Confirmation of snoring changes in positional OSA patients is required. In this study, only patients with positional OSA were included. We aimed to identify correlations of changes in snoring sounds when patients changed their sleep position (from supine to lateral) with the AHI and other parameters. We also measured the formant frequency of snoring sounds by sleep position.
Patient medical histories were obtained and physical examinations were performed prior to overnight polysomnography (PSG). During PSG, snoring sounds were recorded for subsequent analysis.
Patients complaining of snoring and OSA, who underwent physical examination and overnight PSG in our sleep clinic from July 2017 to May 2018, were enrolled in this study. We also calculated the body mass index (BMI) and examined the nasal and oral cavities for anatomical variations that might affect airflow. Patients who had undergone previous airway surgery, had central sleep apnea, or exhibited muscular or joint injuries in the head-and-neck region were excluded. We enrolled 18 males and 1 female diagnosed with positional OSA on overnight PSG (Table 1). The research protocol was reviewed and approved by our Institutional Review Board (IRB approval number 2018-11).
Overnight PSG (EMBLA Embletta MPR-PG; Natus Medical, Pleasanton, CA, USA) was performed for each patient by the same PSG specialist. Electroencephalography, bilateral electro-oculography, and submental electromyography were used to determine sleep stage. Oronasal airflow was monitored by a thermistor. Thoracoabdominal respiratory effort was measured using a respiratory sensor placed over the rib case and abdomen. Oxyhemoglobin saturation was recorded using a finger pulse oximeter. Body position during sleep, recorded by a sensor, was classified into five categories: supine, right and left lateral, prone, or upright. All data were recorded on a personal computer.
Once PSG had commenced, we recorded all snoring sounds and body positions. A microphone was suspended 1.5 m above the bed. We recorded three snoring sounds immediately after apnea, which were analyzed phonetically and averaged. The sounds were digitized and edited according to sleep stage [non-rapid eye movement (NREM) vs. rapid eye movement (REM)] and sleep position (supine vs. both lateral positions). The sounds were analyzed using Praat software (ver. 5.2.16; http://www.praat.org). The sampling rate was converted to 44100 Hz. We measured differences in sound intensity (dB), spectrographs, and formant frequencies between patients in the supine and both lateral positions.
We used a linear regression model and the paired t-test to assess correlations among changes in snoring intensity, the AHI, and formant frequencies. All tests were performed using SPSS software (ver. 25.0; IBM Corp., Armonk, NY, USA). A p-value<0.05 was considered to indicate statistical significance.
Table 2 shows the snoring intensity by sleep position and other parameters. We found no significant correlation of snoring intensity with the AHI, respiratory disturbance index (RDI), BMI, or neck circumference. Next, we assessed changes in snoring according to a change in sleep position, from supine to lateral. In general, the snoring intensity decreased when the sleep position changed from supine to lateral. Five patients whose snoring completely disappeared were excluded from the analysis. We found significant negative correlations of the AHI and RDI with difference of snoring sound intensity when sleep position changed from supine to lateral position. Lower AHI and RDI values in positional OSA patients were associated with a larger decrease in snoring when the sleep position changed from supine to lateral (Table 3, Fig. 1).
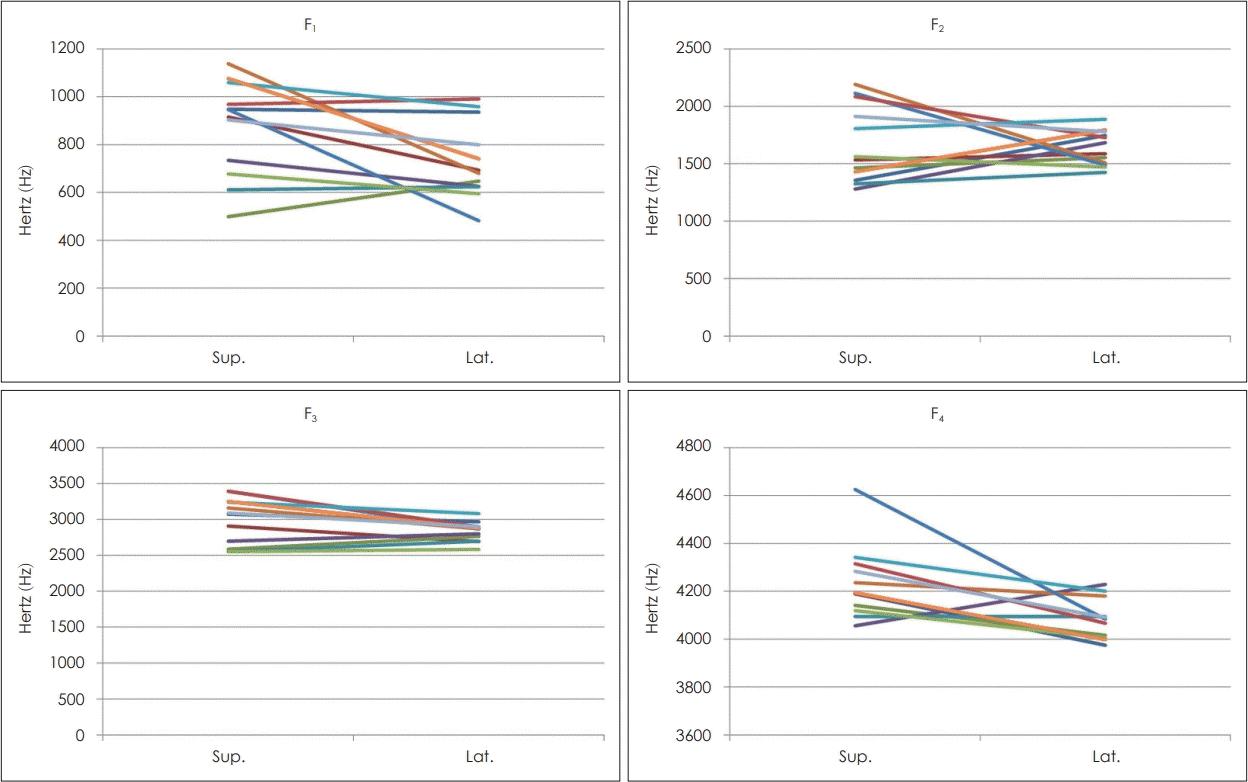
Of the 19 patients, the snoring sounds of 13 were analyzed in terms of formant frequency; 5 patients whose snoring disappeared completely, and 1 whose snoring sounds could not be analyzed because of a recording error, were excluded from the analysis. A paired t-test was performed to confirm the change in each formant frequency according to the change in sleep position (supine to lateral). Formant frequencies 1 (F1), F3, and F4 were significantly decreased (p-value: 0.02, 0.03, and 0.01, respectively). The reduction in F2 was not statistically significant (Table 4). Fig. 2 shows the frequency change in each patient.
The first report of OSA therapy based on sleep position appeared in 1982 [12]. Many subsequent studies confirmed that a change in sleep position from supine to lateral reduced the incidence of respiratory events [13-19]. Patients with an increase in the rate of respiratory events (apnea or hypopnea) at least two-fold higher in the supine than in the lateral position are diagnosed with positional OSA [20]; this type of OSA accounts for more than half of all OSA cases [21]. To the best our knowledge, this study is the first to describe changes in snoring and formant frequency when patients with positional OSA change their sleep position. Koutsourelakis, et al. [22] showed that snoring tended to be louder during NREM than REM sleep. In particular, the snoring time was longest, and the snoring intensity highest, in NREM stage 3 (N3) [11]. When the sleep posture changed from supine to lateral, snoring disappeared in 5 patients, decreased in 10, and increased in 3. All patients whose snoring disappeared had AHIs of less than 15, and all those whose snoring increased had AHIs greater than 40. Snoring tended to decrease when patients with AHIs of 15-40 assumed a lateral position (the lower the AHI, the greater the decrease). Nakano, et al. [11] described an effect of body position on snoring in apneic and nonapneic snorers. Nonapneic (but not apneic) snoring intensity decreased when the sleep position changed from supine to lateral, where the cutoff for distinguishing nonapneic from apneic snoring was an AHI of 15. We found that snoring intensity decreased when positional OSA patients changed their sleep position from supine to lateral, with reductions occurring in all moderate OSA patients (15<AHI<30) and in some severe OSA patients (30<AHI<40). Indeed, in mild OSA patients (AHI<15), snoring disappeared, as also found by Nakano, et al. [11] However, in patients with an AHI exceeding 40, snoring intensity and the AHI were not correlated; in some patients, snoring increased in a lateral sleep position. Lee, et al. [3] showed that the principal upper airway obstruction site varied by sleep position in OSA patients. In the supine position, the soft palate and tongue base constituted the principal obstruction site, whereas in the lateral position, it was the lateral pharyngeal wall. Patients with an AHI of 15-30 exhibited less severe lateral pharyngeal wall obstruction in the lateral position, but the obstruction increased in those with an AHI exceeding 30. Yalamanchili, et al. [23] found that the oropharyngeal wall was the principal obstruction site in OSA patients, especially when sleeping in the lateral position. Thus, changes in sleep position may affect the principal upper airway obstruction site. The lateral wall makes a major contribution to obstruction in the lateral position.
We examined changes in snoring sound frequency by sleep position. Speech involves both voiced and unvoiced sounds, whereas snoring is unvoiced, as the sound is created in the laryngeal and supralaryngeal regions, not by the vocal folds [24]. In addition, the airway affects the energy transfer at a particular frequency. The resonance frequency allowing the maximal energy transfer is the formant frequency (F) [25]. However, because snoring sounds are caused by pharyngeal structures, not the vocal cords, speech analysis techniques are of limited use. Nevertheless, the formant frequency has been determined in several studies that analyzed the acoustic characteristics of snoring sounds, such that the same analysis was performed in the present study [24-26].
F1 reflects the extent of pharyngeal constriction and the height of the tongue. F2 is related to the degree of advancement of the tongue relative to its neutral position, where the frequency increases as the retrolingual space increases. F3 correlates with the degree of lip-rounding. F4 is related to the location and shape of the larynx and laryngeal ventricle, but there is as yet no general consensus on the precise relationship [24-28]. F1 and F2 can be used to distinguish among the vowels, whereas F3 and F4 are related to the individual timbre.
We evaluated the change in formant frequency (F1-F4) according to the change in sleep position, except in patients whose snoring disappeared in the lateral position. Statistically significant decreases were determined in F1, F3, and F4. These results indicate that a change in sleep position from supine to lateral increases the tongue height, widens the pharynx and causes a change in lip shape to non-circular. In the case of F2, the frequency increased in patients with decreased snoring intensity and decreased in those with increased snoring intensity (147.51 vs. -439.85 Hz), although the changes were not statistically significant. These observations suggest that when the sleep position is changed from supine to lateral in those with decreased snoring intensity, the position of the tongue is advanced relative to the neutral position and the retrolingual space is widened. However, according to the formula used to obtain the formant frequency, Fn=(2n-1)C/4L (where n is a number from 1-4, Fn is the formant frequency, C is the velocity of sound in the air, and L is the length of vocal tract), the change in sleep position causes a change in the vibrating site of the upper airway where the snoring sound is created, which in turn changes L.
The principal limitation of this study was that relatively few patients were included; therefore, we could not stratify the analyses by AHI. OSA is classified as mild, moderate or severe based on the AHI. We could not statistically compare changes in snoring intensity and formant frequency between OSA subgroups, nor explore gender differences, due to the small number of patients. Also, the time and intensity of snoring sounds are known to differ depending on the sleep stage. In a previous study, N3 had the longest snoring time, followed by N2, REM, and N1. N3 also had the largest snoring intensity, followed by N2, N1, and REM [11. However, a limitation of our study was that the NREM stage was not further stratified.
OSA treatment is initially conservative, and includes weight loss and cessation of alcohol intake followed by continuous positive airway pressure therapy, oral appliance therapy, and sleep surgery [29,30]. Positional OSA patients can also use devices that prohibit the supine sleep position. However, our data showed that although positional therapy may reduce the incidence of respiratory events in OSA patients, the effect on snoring may differ depending on the AHI and RDI. Therefore, when applying positional therapy, it should be recognized that its purpose is not to decrease snoring, but rather to decrease the incidence of respiratory events.
In conclusion, when the sleep posture changed from supine to lateral in patients with positional OSA, snoring sounds were reduced, with the extent of the reduction depending on the AHI, and the formant frequency decreased.
Notes
Author Contribution
Conceptualization: Soo Kweon Koo. Data curation: Ho Byung Lee, Chang Lok Ji, Geun Hyung Park, Sang Jun Lee. Formal analysis: Tae Kyung Koh, Soon Bok Kwon, Sang Jun Lee. Methodology: Soon Bok Kwon, Ho Byung Lee, Geun Hyung Park. Project administration: Tae Kyung Koh. Supervision: Soo Kweon Koo. Writing— original draft: Ho Byung Lee, Chang Lok Ji. Writing—review & editing: Tae Kyung Koh, Ho Byung Lee.
REFERENCES
1. Acar M, Yazıcı D, Bayar Muluk N, Hancı D, Seren E, Cingi C. Is there a relationship between snoring sound intensity and frequency and OSAS severity? Ann Otol Rhinol Laryngol. 2016; 125(1):31–6.

2. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008; 5(2):136–43.

3. Lee CH, Kim DK, Kim SY, Rhee CS, Won TB. Changes in site of obstruction in obstructive sleep apnea patients according to sleep position: A DISE study. Laryngoscope. 2015; 125(1):248–54.

4. Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, Duran-Cantolla J, Peña Mde L, Masdeu MJ, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013; 187(1):99–105.

5. Namtvedt SK, Hisdal J, Randby A, Agewall S, Stranden E, Somers VK, et al. Impaired endothelial function in persons with obstructive sleep apnoea: Impact of obesity. Heart. 2013; 99(1):30–4.

6. Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, Soler-Cataluña JJ, Almeida-Gonzalez C, De la Cruz Morón I, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: Role of long-term continuous positive airway pressure treatment: A prospective observational study. Am J Respir Crit Care Med. 2012; 186(9):909–16.
7. Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012; 5(5):720–8.
8. Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012; 307(20):2169–76.

9. Hrubos-Strøm H, Einvik G, Nordhus IH, Randby A, Pallesen S, Moum T, et al. Sleep apnoea, anxiety, depression and somatoform pain: A community-based high-risk sample. Eur Respir J. 2012; 40(2):400–7.

10. American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999; 22(5):667–89.
11. Nakano H, Ikeda T, Hayashi M, Ohshima E, Onizuka A. Effects of body position on snoring in apneic and nonapneic snorers. Sleep. 2003; 26(2):169–72.

12. Jackson EI. Schmidt HS. Modification of sleeping position in the treatment of obstructive sleep apnea. Sleep Res. 1982; 11:149.
13. Cartwright RD, Lloyd S, Lilie J, Kravitz H. Sleep position training as treatment for sleep apnea syndrome: A preliminary study. Sleep. 1985; 8(2):87–94.

14. Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999; 115(3):771–81.

15. Skinner MA, Kingshott RN, Jones DR, Homan SD, Taylor DR. Elevated posture for the management of obstructive sleep apnea. Sleep Breath. 2004; 8(4):193–200.

16. Zuberi NA, Rekab K, Nguyen HV. Sleep apnea avoidance pillow effects on obstructive sleep apnea syndrome and snoring. Sleep Breath. 2004; 8(4):201–7.

17. Heinzer RC, Pellaton C, Rey V, Rossetti AO, Lecciso G, Haba-Rubio J, et al. Positional therapy for obstructive sleep apnea: An objective measurement of patients’ usage and efficacy at home. Sleep Med. 2012; 13(4):425–8.

18. van Maanen JP, Richard W, Van Kesteren ER, Ravesloot MJ, Laman DM, Hilgevoord AA, et al. Evaluation of a new simple treatment for positional sleep apnoea patients. J Sleep Res. 2012; 21(3):322–9.

19. van Maanen JP, de Vries N. Long-term effectiveness and compliance of positional therapy with the sleep position trainer in the treatment of positional obstructive sleep apnea syndrome. Sleep. 2014; 37(7):1209–15.

20. Frank MH, Ravesloot MJ, van Maanen JP, Verhagen E, de Lange J, de Vries N. Positional OSA part 1: Towards a clinical classification system for position-dependent obstructive sleep apnoea. Sleep Breath. 2015; 19(2):473–80.

21. Cartwright R, Ristanovic R, Diaz F, Caldarelli D, Alder G. A comparative study of treatments for positional sleep apnea. Sleep. 1991; 14(6):546–52.

22. Koutsourelakis I, Perraki E, Zakynthinos G, Minaritzoglou A, Vagiakis E, Zakynthinos S. Clinical and polysomnographic determinants of snoring. J Sleep Res. 2012; 21(6):693–9.

23. Yalamanchili R, Mack WJ, Kezirian EJ. Drug-induced sleep endoscopy findings in supine vs nonsupine body positions in positional and nonpositional obstructive sleep apnea. JAMA Otolaryngol Head Neck Surg. 2019; 145(2):159–65.

24. Ng AK, Koh TS, Baey E, Lee TH, Abeyratne UR, Puvanendran K. Could formant frequencies of snore signals be an alternative means for the diagnosis of obstructive sleep apnea? Sleep Med. 2008; 9(8):894–8.

25. Koo SK, Kwon SB, Moon JS, Lee SH, Lee HB, Lee SJ. Comparison of snoring sounds between natural and drug-induced sleep recorded using a smartphone. Auris Nasus Larynx. 2018; 45(4):777–82.

26. Won TB, Kim SY, Lee WH, Han DH, Kim DY, Kim JW, et al. Acoustic characteristics of snoring according to obstruction site determined by sleep videofluoroscopy. Acta Otolaryngol. 2012; 132(Suppl 1):S13–20.

28. Koo SK, Lee SH, Chon KM, Wang SG, Goh EK, Kim HJ, et al. Mechanism of vocal phonation in T-E shunt patients after total laryngectomy. Korean J Otolaryngol. 1998; 41(3):360–70.
Fig. 1.
Correlations between changes in snoring and the RDI and AHI. RDI: respiratory disturbance index, AHI: apnea-hyponea index, Sup.: supine position, Lat.: lateral position, ΔSnoring intensity: change in snoring intensity from the supine to the lateral position.
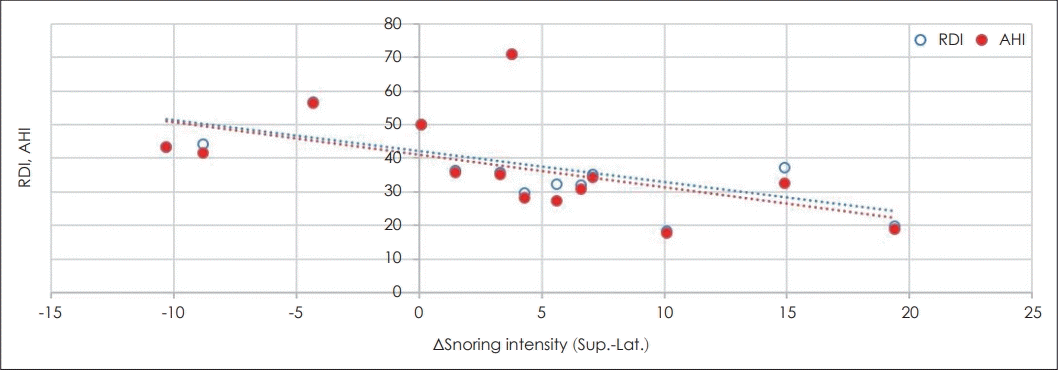
Table 1.
Demographic characteristics of the patients (n=19; 18 males and 1 female)
Table 2.
Body and sleep parameters
Table 3.
Correlations between changes in snoring intensity and changes in sleep position
| Parameter | β | p-value | |
|---|---|---|---|
| ΔSnoring intensity (dB) | RDI | -0.719 | 0.013 |
| AHI | -0.699 | 0.015 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download