Abstract
Background and Objectives
We investigated whether the intratympanic steroid injections might be an effective initial treatment for sudden sensorineural hearing loss (SSNHL) in patients with diabetes mellitus (DM).
Subjects and Method
We assessed a total of 167 patients with DM and SSNHL who visited a tertiary referral otolaryngology department between January 2010 and April 2018. Forty-two patients with DM and SSNHL received intratympanic steroid injections; 48 patients with DM and SSNHL received systemic steroid treatment; and 77 patients with DM and SSNHL received a combination of systemic and intratympanic treatment. Initial and post treatment hearing levels and fasting blood sugar (FBS) were assessed, and correlations between hearing gain and the duration of DM, HbA1c, FBS were investigated.
Results
After steroid treatment, hearing levels were 38.87±25.35 dB in the intratympanic injection group, 41.09±28.49 dB in the systemic steroid treatment group, and 47.81±27.12 dB in the combined treatment group. Final hearing levels and hearing gain in the three groups did not differ significantly. FBS after treatment in the systemic steroid treatment and combined steroid treatment group worsened relative to the intratympanic injection group (202.00±9.40 mg/dL to 326.63±7.85 mg/dL). FBS, duration of DM, and HbA1c levels did not affect the hearing gain in patients with DM and SSNHL.
Sudden sensorineural hearing loss (SSNHL) is an otologic emergency. SSNHL is commonly defined as hearing loss of greater than 30 dB involving at least three consecutive frequencies and occurring within a 3-day period [1]. There are many hypotheses regarding the etiology of SSNHL, including those implicating viral infection, vascular compromise, and immune-mediated responses [2]. Patients diagnosed within 1-2 weeks of symptom onset have the most favorable prognosis as SSNHL requires urgent treatment for the recovery of auditory function [3], Various treatments for SSNHL have been employed, including steroids [4], antiviral therapy [5], plasma expanders [6], vasodilators [7], and hyperbaricoxygenation [8].
High dose steroid administration is currently the mainstay treatment for SSNHL. However, the use of systemic steroids can worsen glycemic control in patients with diabetes mellitus (DM). Additionally, the disease duration of DM may contribute to microvascular problems, which can affect the delivery and efficacy of systemic steroids in the cochlea [9]. In line with this, histological study of the temporal bones in DM patients reveals stria vascularis damage [10]. Given this, systemic steroid use in patients with DM and SSNHL may lead to poorer outcomes than are observed in those without DM [11]. Intratympanic steroid injections are used as additional treatment following systemic steroid therapy in SSNHL patients. To avoid systemic side effects and increase steroid efficacy in the cochlea, local intratympanic steroid injections into the middle ear may serve as an excellent treatment alternative.
In the present study, we compared sensorineural hearing outcomes following intratympanic, systemic, and combined steroid therapy in individuals with DM. The results of the present study may thus impact clinical treatment decisions concerning steroid treatment in individuals with SSNHL and DM.
Patients presenting to the Soonchunhyang University Bucheon Hospital’s Department of Otorhinolaryngology-Head and Neck Surgery between January 2010 and April 2018 with a history of DM and SSNHL were retrospectively reviewed. All patients in the present study exhibited unilateral SSNHL of at least 30 dB across at least three frequencies and occurring within 3 days of hearing loss. All patients treated at admission or during outpatient follow-up were included. Patients with a history of previous SSNHL, previous otologic surgery on the affected ear, an identifiable cause for SSNHL, or an acute or chronic otitis media of the affected ear were excluded. Participant’s sex, age, elapsed time preceding treatment, symptoms of dizziness, degree of the hearing loss, HbA1c, and any additional complications were also recorded. The authors had no ethical concerns in performing this retrospective study. This study was approved by the Institutional Review Board (IRB No. 2019-02-003) of Soonchunhyang University.
A total of 167 patients were included, given the selection criteria outlined above. These were divided into three groups based on their steroid administration status: 48 patients received oral or intravenous steroids, 42 underwent intratympanic injections, and 77 patients were treated by a combination of systemic steroids and intratympanic steroid injections.
If patients could be treated in hospital by admission, systemic steroid was used. For patients who could not be admitted on hospital, intratympanic dexamethasone injection was used to treat them. If hearing gain was not seen after intratympanic dexamethasone injection, patients were recommended to be admitted to the hospital and receive systemic steroid treatment. Also, intratympanic dexamethasone injection additionally performed in patients who did not show improvement in their hearing function despite treatment with systemic steroid.
Patients in the intratympanic steroid injection group (n=42) received intratympanic dexamethasone injections (0.2-0.5 mL of 5 mg/mL dexamethasone phosphate disodium, 5 mg/mL, Ilsung Pharmaceuticals CO., Seoul, Korea) for 3-7 days (three to five injections in total). As half time of dexamethasone is 36-54 hours, patients received intratympanic dexamethasone injection every day or at least two days interval and most patients received three injections in total [12].
This procedure was performed in the supine position with the head tilted 30-45° towards the healthy ear. After positioning the patient, an anterosuperior puncture was made in the tympanic membrane (visualized with a microscope) with a 25-gauge needle and a 1 cc syringe. Patients were instructed to avoid swallowing, talking, or changing their position for 20 min following the injection.
The systemic steroid treatment group (n=48) were administered either oral Methylon (methylprednisolone, 4 mg/tab, ALVOGENKOREA, Seoul, Korea) for 10 days or intravenous systemic dexamethasone (dexamethasone phosphate disodium, 5 mg/mL) for 7 days. Some patients used both oral medication and systemic injections. Patients who were treated orally took 6 tabs (24 mg) twice daily (48 mg in total) for 5 days. They then took 4 tabs (16 mg) twice daily (32 mg in total) for the next 2 days. On 8th and 9th days of treatment, they took 2 tabs (8 mg each) twice daily (16 mg total). On the last day of treatment, patients were instructed to take 2 tabs (8 mg) at one time. Patients who received intravenous dexamethasone were exposed to a dose of 10 mg/day for 5 days and then 5 mg/day for 2 days. After discharge, they took oral tabs for 3 days, just as those who used oral methylon exclusively.
Finally, 77 patients were treated with a combination of systemic and intratympanic steroid injections. This group includes patients who either received intratympanic steroid injection followed by systemic steroid treatment or systemic steroid treatment followed by intratympanic steroid injection due to no improvement in the hearing level by initial treatment.
Pure tone audiometry (PTA) was performed in all patients at both initial intake and following treatment. PTA was calculated as the average hearing thresholds at four frequencies (0.5, 1, 2, and 4 kHz). Hearing improvement was evaluated according to Siegel’s criteria [13]. ‘Complete recovery’ was defined as more than 30 dB of hearing gain and final hearing threshold of better than 25 dB. ‘Partial recovery’ was defined as more than 15 dB of hearing gain and final hearing threshold between 25 and 45 dB. ‘Slight improvement’ was defined as more than 15 dB of hearing gain but and a final hearing threshold below 45 dB. Finally, ‘no improvement’ was defined as less than 15 dB of hearing gain and a final hearing threshold below 75 dB.
Characteristics of the three groups are presented as means±standard deviations for continuous variables. The differences between groups were analyzed using analysis of variance (ANOVA) testing and X2 tests to compare hearing recovery among the three groups. Sex and dizziness symptoms were analyzed by X2 tests and other factors were analyzed by ANOVA. SPSS version 24.0 (IBM Corp., Armonk, NY, USA) was used for all analyses. Significance was indicated by a p-value <0.05.
The 167 patients assessed here were divided into systemic (n=48; 28 males and 20 females), intratympanic (n=42; 22 males and 20 females), and combined treatment groups (n=77; 40 males and 37 females). The mean age of patients who underwent systemic steroid therapy, intratympanic, or combined therapy differed significantly from one another and were 58.41±12.75, 62.28±10.29, and 58.78±10.97 years, respectively. Additionally, initial hearing levels and initial speech discrimination scores (SDS) among combined therapy group patients were significantly higher than in the other two groups. The other characteristics assessed did not significantly differ among the groups. Patient characteristics are summarized in Table 1.
Final hearing outcomes are based on Siegel’s criteria. ‘Complete recovery,’ ‘partial recovery,’ and ‘slight improvement’ were considered to indicate recovery of hearing function. In the systemic administration group, 54.17% recovered hearing function, and the complete, partial recovery, and slight improvement rates were 35.42, 12.5, and 6.25%, respectively. The total hearing improvement rate was 58.44% in the combined treatment group and 50% in the intratympanic group. Differences in hearing recovery rates (Siegel’s criteria I+II+III) among the groups did not differ significantly (p=0.668).
Pretreatment hearing levels were significantly different among the three groups. They were 56.51±29.15 dB in the systemic administration group, 69.98±26.78 dB in the combined group, and 51.29±26.99 dB in the intratympanic group (p=0.001) (Fig. 1). All three groups exhibited statistically significant hearing gain with treatment (p<0.001; in all groups). Posttreatment hearing levels were 41.09±28.49 dB in the systemic administration group, 47.81±27.12 dB in the combined group, and 38.87±25.35 dB in the intratympanic group (p=0.177). Although initial hearing levels in the combined group were higher than in any other group, the difference in the posttreatment hearing levels was not statistically significant. Similarly, hearing gain in the three groups did not differ significantly: 15.42±22.66 dB gained in the systemic administration group, 22.16±24.0 dB in the combined group, and 12.42±17.53 dB in the intratympanic group) (p=0.053).
Fasting blood sugar (FBS) was measured in systemic administration group and combined group. The initial average FBS level was 202.0±9.4 mg/dL and the FBS after treatment was significantly increased to 326.6±7.9 mg/dL (p<0.001) (Fig. 2).
FBS, duration of DM, and HbA1c levels were analyzed for correlations with hearing gain in all 167 patients with DM and SSNHL. The average hearing gain in the three groups was 17.77±22.48 dB. Additionally, a linear regression analysis revealed no statistically significant correlation between FBS and hearing gain (p=0.261). The mean duration of DM in the three groups was 8.68±7.32 years. There was no significant correlation between the duration of DM and hearing gain (p=0.832). A linear regression analysis additionally revealed no statistically significant correlation between HbA1c levels and hearing gain (p=0.327).
Several treatments have been reported for SSNHL including use of vasoactive substances, hyperbaric oxygen, antivirals, and vitamins [2,5,6]. The efficacy of each approach has, however, been debated, with no standard, universally accepted treatment apart from steroid therapy [2]. Systemic corticosteroids have been the mainstay of treatment for SSNHL since Wilson’s 1980 prospective, randomized controlled trial that demonstrated that oral steroids led to superior hearing outcomes than a placebo [14]. However, patients with DM, hypertension, gastric ulcers, tuberculosis, or those who are pregnant also face potential side effects following systemic steroid use [11,15]. As an alternative to broader, systemic treatments, in 1996, Silverstein et al. [16] introduced intratympanic steroid application via the round window membrane, which is associated with fewer side effects. Intratympanic steroid injections have also been made available in the outpatient setting and can be administered easily. Recently, intratympanic steroid therapy has also begun to be considered a second-line therapy for SSNHL, following systemic steroid use [17].
Although the mechanism underlying the efficacy of steroids for treatment of SSNHL remains unclear, the anti-inflammatory, immunosuppressive, and electrolyte balancing functions of steroids may underlie any potential mechanisms. Steroids suppress the activation of the transcription factor nuclear factor-kappa beta, which is necessary for the production of immune cells and factors. Moreover, steroids serve to decrease circulating immune complexes which are an indication of increased levels of self-reactive T and B lymphocytes [18]. The anti-inflammatory functions of steroids are due to, among other things, their weakening of neutrophils adherence to capillary endothelial cells by blocking the expression of adhesion molecules and various chemokines [19]. Additionally, steroids regulate sodium and potassium ion transport via activation of mineralocorticoid receptors, thus aiding in restoration of inner ear homeostasis [20].
For patients with DM, steroid treatment may be administered via either systemic (oral pill or intravenous injection) or intratympanic injections, as has been noted above. In fact, systemic steroids are widely used for their anti-inflammatory and immunosuppressive effects. However, Ermutlu et al. [21] reported that the effectiveness of systemically administered steroids may by decreased by their limited permeability through the blood-perilymphatic barrier. In patients who have had diabetes for many years, angiopathies in the blood vessels supplying the inner ear may occur. As the cochlea is known to be particularly vulnerable to microvascular circulation alterations, diabetic angiopathy may damage the inner ear by occluding its blood supply [11]. Along these same lines, Jorgensen [10] study of several animal and human temporal bones revealed thickened and damaged capillary walls of the stria vascularis with diabetes. While thickened capillary walls cause decreased microcirculation in the cochlea, cellular damage accompanied by hypoxia also occurs and eventually results in hearing loss [22]. In another study of 50 patients with DM, Karabulut et al. [23] reported outer hair cell damage and that transient evoked otoacoustic emissions amplitudes at 1, 1.5, 2, 3, 4, and 6 kHz featured lower signal to noise ratio amplitudes on distortion product otoacoustic emissions in DM patients. Given these collective findings, we posit that intratympanic steroid injections may offer the advantage of lower risk for potential side effects while directly affect inner ear function via diffusion through the round window, resulting in increased perilymph steroid concentrations [11,21]. In patients with DM, intratympanic steroid treatments may serve as the preferred first-line treatment for SSNHL.
The results discussed here reveal that intratympanic and systemic steroid administration may be useful treatments in patients with DM and SSNHL. This is in accordance with the majority of the literature, in which most reports found that intratympanic steroids demonstrated better or at least similar hearing gain and less complications compared to systemic steroid treatment [11,15]. For instance, Kakehata et al. [15] reported a recovery rate of 70% among intratympanic steroid users when steroids were administered following a myringotomy or via a tympanostomy tube and a rate of 67% among systemic steroid users. Critically however, some patients in the latter group exhibited exacerbated hyperglycemia with systemic steroid use. Similarly, Han et al. [11], in their study of 114 patients, found that all peroral administration (n=48), intravenous administration (n=32), and intratympanic administration (n=34) group participants experienced improved hearing, with no significant differences in hearing gain or recovery among the groups. Aside from three patients in the systemic steroid group who discontinued treatment due to hyperglycemia, all patients who were administered intratympanic steroid treatments experienced good control of their blood sugar levels. These data, together with our own, indicate that intratympanic steroid treatments are at least as effective, if not more effective, than systemic steroid treatment of SSNHL in patients with DM.
The present study has several limitations. First, glucose level was not checked in intratympanic steroid treatment group. Second, since this was a retrospective study, some of the factors including the initial hearing level, initial SDS and first steroid administration day were stastically different among 3 groups which could have affected the hearing outcome. As our retrospective study compared 3 groups affected by those factors, it could show statistically different results with prospective study. However the present study retrospectively investigated large number of patients, and included combined steroid treatment group. Further prospective studies need to be performed to clarify the efficacy of intratympanic steroid injection for SSNHL in patients with DM.
Intratympanic steroid injections are comparable in efficacy to other steroid treatment modalities for the treatment of SSNHL in patients with DM. Furthermore, localized intratympanic treatments offer the advantage of minimal side effects in patients with DM. Although our study has some limitations, the present study also found that intratympanic steroid injections were as effective as systemic steroid therapy or combined (intratympanic and systemic) steroid therapy. We thus conclude that intratympanic steroid injections may serve as an initial and preferred treatment modality for SSNHL in patients with DM.
REFERENCES
1. Seggas I, Koltsidopoulos P, Bibas A, Tzonou A, Sismanis A. Intratympanic steroid therapy for sudden hearing loss: a review of the literature. Otol Neurotol. 2011; 32(1):29–35.
2. Zhao D, Tong B, Wang Q, Hellstrom S, Duan M. A comparison of effects of systemic and intratympanic steroid therapies for sudden sensorineural hearing loss: A meta-analysis. J Otol. 2016; 11(1):18–23.

3. Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008; 359(8):833–40.
4. Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch Otolaryngol. 1980; 106(12):772–6.

5. Shaikh JA, Roehm PC. Does addition of antiviral medication to high-dose corticosteroid therapy improve hearing recovery following idiopathic sudden sensorineural hearing loss? Laryngoscope. 2011; 121(11):2280–1.

6. Klemm E, Bepperling F, Burschka MA, Mösges R, Study G. Hemodilution therapy with hydroxyethyl starch solution (130/0.4) in unilateral idiopathic sudden sensorineural hearing loss: A dosefinding, double-blind, placebo-controlled, international multicenter trial with 210 patients. Otol Neurotol. 2007; 28(2):157–70.
7. Ogawa K, Takei S, Inoue Y, Kanzaki J. Effect of prostaglandin E1 on idiopathic sudden sensorineural hearing loss: A double-blinded clinical study. Otol Neurotol. 2002; 23(5):665–8.

8. Liu SC, Kang BH, Lee JC, Lin YS, Huang KL, Liu DW, et al. Comparison of therapeutic results in sudden sensorineural hearing loss with/without additional hyperbaric oxygen therapy: A retrospective review of 465 audiologically controlled cases. Clin Otolaryngol. 2011; 36(2):121–8.

10. Jorgensen MB. The inner ear in diabetes mellitus. Histological studies. Arch Otolaryngol. 1961; 74:373–81.

11. Han CS, Park JR, Boo SH, Jo JM, Park KW, Lee WY, et al. Clinical efficacy of initial intratympanic steroid treatment on sudden sensorineural hearing loss with diabetes. Otolaryngol Head Neck Surg. 2009; 141(5):572–8.

12. Maksoud A, Hassan D, Nafie Y, Saadb A. Intratympanic dexamethasone injection in Meniere’s disease. Egypt J Otolaryngol. 2015; 31(2):128–34.

13. Siegel LG. The treatment of idiopathic sudden sensorineural hearing loss. Otolaryngol Clin North Am. 1975; 8(2):467–73.

14. Hobson CE, Alexander TH, Harris JP. Primary treatment of idiopathic sudden sensorineural hearing loss with intratympanic dexamethasone. Curr Opin Otolaryngol Head Neck Surg. 2016; 24(5):407–12.

15. Kakehata S, Sasaki A, Oji K, Futai K, Ota S, Makinae K, et al. Comparison of intratympanic and intravenous dexamethasone treatment on sudden sensorineural hearing loss with diabetes. Otol Neurotol. 2006; 27(5):604–8.

16. Silverstein H, Choo D, Rosenberg SI, Kuhn J, Seidman M, Stein I. Intratympanic steroid treatment of inner ear disease and tinnitus (preliminary report). Ear Nose Throat J. 1996; 75(8):468–71.

17. Ho HG, Lin HC, Shu MT, Yang CC, Tsai HT. Effectiveness of intratympanic dexamethasone injection in sudden-deafness patients as salvage treatment. Laryngoscope. 2004; 114(7):1184–9.
18. Trune DR, Kempton JB, Harrison AR, Wobig JL. Glucocorticoid impact on cochlear function and systemic side effects in autoimmune C3.MRL-Faslpr and normal C3H/HeJ mice. Hear Res. 2007; 226(1-2):209–17.

19. Skubitz KM, Craddock PR, Hammerschmidt DE, August JT. Corticosteroids block binding of chemotactic peptide to its receptor on granulocytes and cause disaggregation of granulocyte aggregates in vitro. J Clin Invest. 1981; 68(1):13–20.

20. Trune DR, Kempton JB, Gross ND. Mineralocorticoid receptor mediates glucocorticoid treatment effects in the autoimmune mouse ear. Hear Res. 2006; 212(1-2):22–32.

21. Ermutlu G, Süslü N, Yılmaz T, Saraç S. Sudden hearing loss: An effectivity comparison of intratympanic and systemic steroid treatments. Eur Arch Otorhinolaryngol. 2017; 274(10):3585–91.

Fig. 1.
Comparison of the hearing recovery among systemic, combined (systemic and intratympanic), intratympanic group. All three groups exhibited statistically significant hearing gain with treatment (p<0.001; in all groups). Although initial hearing levels in the combined group were higher than in any other group, the difference in the posttreatment hearing levels was not statistically significant. Similarly, hearing gain in the three groups did not differ significantly. *p<0.05.
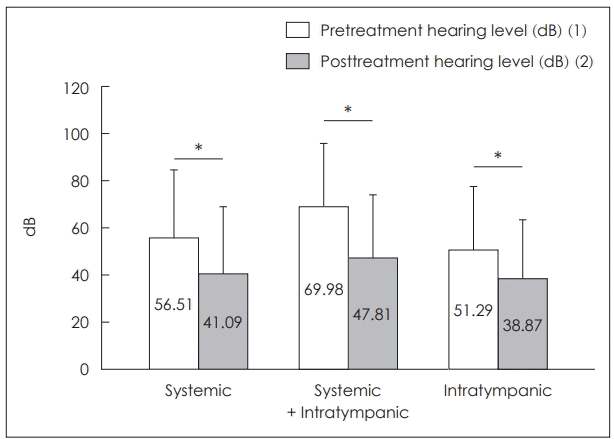
Fig. 2.
FBS level in systemic administration group and combined group. The initial average FBS level was 202.0±9.4 mg/dL and the FBS after treatment was significantly increased to 326.6±7.9 mg/dL. *p<0.05. FBS: fasting blood sugar.
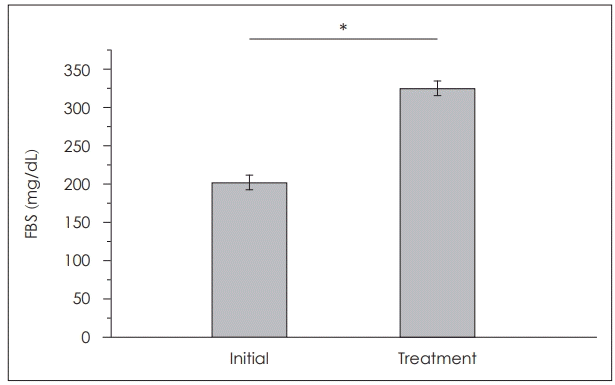
Table 1.
Patient demographics by treatment group
| Variables | Systemic | Systemic+Intratympanic | Intratympanic | p-value |
|---|---|---|---|---|
| Number of patients | 48 | 77 | 42 | |
| Male:female | 28:20:00 | 40:37:00 | 22:20 | 0.765 |
| Age (years) | 58.41±12.75 | 58.78±10.97 | 62.28±10.29 | 0.201 |
| Duration of DM (years) | 8.34±6.41 | 8.38±8.06 | 9.69±6.77 | 0.619 |
| HbA1c (%) | 7.67±1.54 | 7.41±1.35 | 7.1±1.34 | 0.33 |
| FBS (mg/dL) | 204.16±85.60 | 200.79±112.27 | 153.04±76.23 | 0.084 |
| Initial hearing level (dB) | 56.51±29.15 | 69.98±26.78 | 51.29±26.99 | 0.001* |
| Initial SDS (%) | 53.52±39.44 | 33.45±34.25 | 58.02±32.96 | 0.001* |
| Dizziness (%) | 7 (14.58) | 13 (16.88) | 3 (7.14) | 0.331 |
| First steroid administration (days) | 4.35±4.62 | 5.64±6.01 | 8.10±7.08 | 0.013* |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download