Abstract
The objective of this study was to perform a systematic review of the literature for application of intranasal sodium citrate in the patients with olfactory dysfunction to help determine the sodium citrate treatments for this condition. Two authors independently searched the data base (Medline, Scopus, and the Cochrane database) for relevant studies from inception to January 2018. Included studies were randomized controlled studies published in English comparing topical sodium citrate application (treatment group) with saline (control group) in patients who had olfactory dysfunction. Outcomes of interest included the change of olfactory identification and threshold during 2 hours post-treatment. Three studies were enrolled in the meta-analysis. Compared with control group, treatment group did not increase posttreatment score of olfactory identification [standardized mean difference (SMD)=-0.03; 95% confidence interval (CI)= -0.29-0.24; I2=0%] and olfactory threshold (SMD=0.18; 95% CI=-0.09-0.45; I2=0%) significantly. In the degree of pre-post improvement of two outcomes, although treatment group statistically showed the significant improvement in olfactory threshold (SMD=0.30; 95% CI=0.05- 0.55; I2=17%), the clinical significance of this outcome was meaningless. Similarly, there was no significant difference in olfactory identification between two groups (SMD=0.17; 95% CI=-0.11-0.45; I2=22%). Unlike the recent favorable results, our summated results presented the uselessness for the local application of sodium citrate in improving patient’s olfactory function. However, we also had some limitation such as small sample size and inconsistent application methods. Therefore, larger trials and standardized methodology are needed to reach more stronger and exact results.
Olfactory dysfunction is relatively common, affecting approximately 20 to 30 percent of adult [1,2]. Including a temporary loss of the sense of smell, some articles report up to 60% of the population experienced olfactory dysfunction [3]. Numerous clinical reports have suggested that olfactory dysfunction could affect nutritional disturbances, social anxiety and depression which consequently influence the quality of life negatively [4,5]. In particular, if occupation depends on the olfactory function, the olfactory dysfunction becomes crucial obstacle. Recently, as people tends to focus on the quality of life, the number of population who are aware of olfactory dysfunction and visit out-patient clinic for diagnosis and treatment is increasing. However, effective treatment options for physicians are limited [6], and mainly focused on oral steroid or intra nasal steroid administration [3].
Calcium in vertebrate olfactory receptor neurons (ORNs) is involved in both odor-induced excitation and intracellular feedback pathways. The effect of the rise in intracellular calcium is a negative feedback action on various stages of the odor transduction mechanism, which is responsible for the down-regulation of the smell sensitivity such as olfactory adaptation. Based on the facts, previously Panagiotopoulos, et al. [7] assumed that a rise in mucosal calcium could contribute to the consequent influx of calcium inside the cell and reducing mucus free calcium levels might modulate these inhibitory effects and thereby enhance olfaction. They showed that intranasal sodium citrate, calcium sequestrant, in hyposmic patients improved olfactory function. Since his research, there have been several studies which intended to demonstrate the effect of sodium citrate on olfactory dysfunction [8-10]. The objective of this article is to perform a systematic review of the literature for intranasal sodium citrate in the patients with olfactory dysfunction to help determine the efficacy of sodium citrate treatments for this condition.
Studies published in English before December 2017 were identified from MEDLINE, SCOPUS, and the Cochrane Register of Controlled Trials, using the following search terms: “olfaction,” “odor identification,” “odor threshold,” “calcium,” “nasal mucus,” “hyposmia,” “anosmia,” and “sodium citrate.”
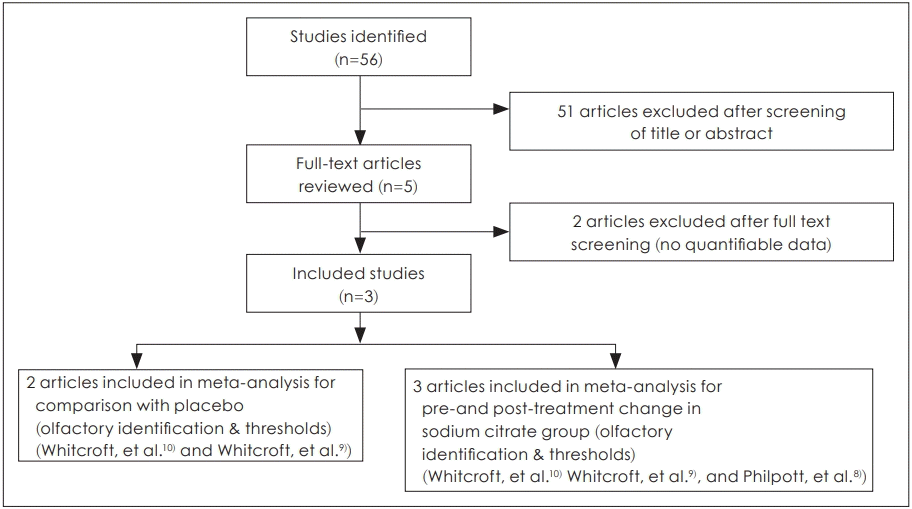
Two reviewers, working independently, screened all abstracts and titles for relevant studies, and discarded any that were not relevant. Randomized controlled trials were eligible for review if they investigated the topical application of sodium citrate in patients who suffered from olfactory dysfunction. Studies were not eligible for inclusion if 1) patients experienced the olfactory dysfunction due to the conductive cause (e.g., nasal polyp or chronic sinusitis); 2) patients had previous history of congenital anosmia; 3) patients suffered from a systemic or malignant disease; or 4) if multiple reports were based on the same trial data. Additionally, in the event of missing or incomplete data, attempts were made to request further details of the published results from the authors directly. Studies were excluded from the analysis if outcomes of interest were not clearly reported with quantifiable data, or if it was not possible to extract and calculate the appropriate data from the published results (Fig. 1).
Data from the eligible studies were extracted using standardized forms and were independently checked by the two reviewers. Sodium citrate, a solution licensed and used safely in other body cavities (e.g., stomach and bladder), is known to buffer calcium ions. Topical application of sodium citrate into olfactory cleft is employed to bind the free calcium in the nasal mucus. It is assumed that sodium citrate reduce the calcium level from nasal mucosa, and subsequent decrease the negative feedback in the olfactory receptor cell response. The primary outcome for this meta-analysis was the posttreatment score of olfactory identification [9,10] or threshold [9,10] within 2 hours after nasal application of sodium citrate. Outcomes associated with sodium citrate after nasal application was compared against a control (placebo). Additionally, the outcomes regarding to change of pre-and post-treatment olfactory identification [9,10] or threshold [8-10] were derived from comparison between the pre- and post-treatment values. The incidence of the adverse effect, a sodium citrate-related morbidity, was assessed as a secondary outcome.
For studies which investigated the influence of sodium citrate on the improvement of olfactory threshold and identification, the following data were extracted: the number of patients, scores of parameters, and p-values recorded in the comparison between the sodium citrate and control groups. The risk of bias for each study was evaluated using the Cochrane “Risk of Bias” tool.
A meta-analysis was performed using “R” statistical software (R Foundation for Statistical Computing, Vienna, Austria). An outcome analysis was performed using the standard mean difference. An effect size of approximately 0.2 is considered a small effect, while 0.5 is considered a medium effect and 0.8 a large and clinically significant effect. A funnel plot and Egger’s test were used simultaneously to detect publication bias. Additionally, the Duval and Tweedie’s trim and fill method was used to adjust for missing studies and to correct the overall effect size according to publication bias. Additionally, sensitivity analyses were performed to estimate the influence of each individual study in the overall meta-analysis results.
Three studies, which included a total of 267 participants, were included and reviewed in this study. Risk of bias assessments and study characteristics are described in Table 1.
In the patients who received sodium citrate (sodium citrate group) and those who received placebo (control group), there were no statistically significantly different in the posttreatment score of olfactory identification [SMD=-0.03; 95% confidence interval (CI)=-0.29-0.24; I2=0%] and olfactory threshold within 2 hours (SMD=0.18; 95% CI=-0.09-0.45; I2=0%) (Fig. 2). Based on the degree of improvement in olfactory identification, there was no significant difference between two groups (SMD=0.17; 95% CI=-0.11-0.45; I2=22%). By contrast, the improvement in olfactory threshold (SMD=0.30; 95% CI=0.05-0.55; I2=17%) was significantly higher in the patients who received sodium citrate (sodium citrate group) than in those who received placebo (control group) (Fig. 3). There was no statistically significant inter-study heterogeneity (I2<50%) in these outcomes. Egger’s test regarding these outcomes was not available due to small number in the enrolled studies. Duval and Tweedie’s trim and fill analysis showed there was no difference between observed and adjusted values. These results showed that the selected studies were not biased.
Sodium citrate did not increase the olfactory identification (SMD=0.02; 95% CI=-0.24-0.29; I2=0%) and olfactory threshold (SMD=0.12; 95% CI=-0.18-0.55; I2=0%) after application significantly. There was no statistically significant inter-study heterogeneity (I2<50%) in these outcomes (Fig. 4). Egger’s test regarding these outcomes was not available due to small number in the enrolled studies. Duval and Tweedie’s trim and fill analysis showed there was no difference between observed and adjusted values. These results showed that the selected studies were not biased.
Sensitivity analyses were performed to evaluate whether the pooled estimates of the change of olfactory threshold and identification were different by omitting a different study each time and repeating the meta-analysis. The results of these sensitivity analyses were all consistent with the above outcomes (data not provided).
Recent studies revealed the role of calcium in olfactory pathway. Odor molecules bind to olfactory receptor cell in the olfactory cleft. G protein-coupled cascade occurs, resulting in increase of cystic adenosine monophosphate (cAMP). cAMP opens cyclic nucleotide-gated channels and then calcium influx occurs. Increase in intracellular calcium acts not only as a second messenger of axon, but also plays inhibitory role. Mucosal calcium gives negative feedback on multiple stages of the olfactory passage [7,11-13]. Through negative feedback, adaptation to long-term odorant exposure is possible. This mechanism could give an interesting idea for a treatment option for olfactory dysfunction by reducing mucosal calcium level and restraining the negative feedback [15,16]. Therefore, there have been som sodium citrate e previous studies which support positive results regarding the administration of functioning as a calcium buffer on olfactory dysfunction.
Assessment of the olfactory function can be generally divided into 3 different components such as odor threshold (the perception of odors at low concentrations), odor discrimination (the nonverbal distinction of different smells), and odor identification (the ability to name or associate an odor). Although there has been no objective assessment of olfactory function in clinical situation, several psychophysical tests assessing olfactory performance have been used for the assessment of each of these components [17]. Sniffin’ Sticks are a validated psychophysical test that is based on pen like odordispensing devices, and previous studies have verified that it shows high test-retest reliability and validity in measuring the olfactory sensitivity [18]. A series of threshold smell tests including phenyl ethyl alcohol was also previously validated and showed the high test-test reliability [19]. This study performed the meta-analysis with previous studies related to sodium citrate and olfactory dysfunction and used the validated psychophysical test to assess the degree of olfactory function. Additionally, the comprehensive evaluation of several components in olfaction, especially including olfactory thresholds, provides the most significant approach to the measurement of olfactory disfunction [17]. Therefore, we measured the olfactory identification and threshold as the olfactory outcomes.
Our results showed that the posttreatment score of olfactory identification and threshold within 2 hours were not significantly changed in sodium citrate group compared with the control group. Although the degree of treatment-related change in olfactory threshold was statistically significantly higher in the sodium citrate group than in control group, the effect size regarding the olfactory threshold was small (SMD=0.3) and there was no significant difference in olfactory identification between two groups. Additionally, in sodium citrate group, the change in olfactory identification and threshold after treatment was also not statistically significantly identified.
The common representation of the SMD is Cohen’s d, which suggests that a smaller effect size indicates that a treatment is clinically less effective. An effect size (SMD) between 2 means of around 0.3 is considered a small effect (possibly clinically non-significant), an effect size of around 0.5 is considered a medium effect, and an effect size of 0.8 or greater is considered a large and clinically significant effect [20]. The only significant effect size for the measurements regarding the treatment-related change in olfactory threshold were no more than 0.3, which meant that these effect sizes were clinically insignificant during treatment periods. These results consistently showed that the application of sodium citrate in patients with olfactory dysfunction would not be helpful to improve their olfaction. In addition, as researchers’ comment, although there were no serious adverse effect related to the application and patient seemed to be tolerable, local trivial adverse effects such as rhinorrhea, nasal obstruction or throat irritation were frequent and the treatment effect short-lived, with only lasting 2 hours [9]. Given the therapeutic effect size and halflife period and frequent minimal side effects, this treatment would not be recommended to the patients clinically.
Panagiotopoulos, et al. [7] firstly performed the pilot case series study and reported that sodium citrate showed the very high effectiveness on the improvement of olfactory function in patients, of which p-value was less than 0.0001. However, since then, the next three randomized controlled studies could not demonstrate as high therapeutic effectiveness as the previous one did, of which p-value were mostly larger than 0.01. Consequently, our studies which included the next three studies could not identify the usefulness of local application of sodium citrate in patients with olfactory dysfunction.
For this discrepancy, there were some reasons. Firstly, Panagiotopoulos, et al. [7] conducted the repeated identification testing on during 3 days. The repeated same test could make participants find the right answer skillfully and the results tend to be biased [9]. Secondly, Panagiotopoulos, et al. [7] used a 12-item identification test, which is less sensitive and specific than the full 16-item test that other researchers utilized [9]. Thirdly, combined testing of two components of olfaction could be more reliable approach to detect the olfactory function and threshold test presented more distinct properties than the identification tests [17]. In previous other studies, olfactory function test included two components such as threshold and identification. However, Panagiotopoulos, et al. [7] concluded on the basis of only identification test. In particular, compared to olfactory threshold, olfactory identification has higher possibility of learning effect [21]. It is possible that learning effect increased identification correction rate as the tests repeats, which may lead to the incorrect results [9]. Fourthly, the effect of calcium in the olfactory function would be more complex than expected previously. In addition to the adaptation of the ORN to odor stimuli (negative) feedback inhibition, all basic transduction processes in the ORN are mediated by calcium. The generation of the receptor action potential, its amplification, and the termination of the receptor potential are the share of calcium itself [22]. Therefore, only depletion of calcium in mucus could explant the olfactory improvements or related outcomes.
Although the results of this study offer the uselessness for the local application of sodium citrate in improving patient’s olfactory function, we also had some limitation. Firstly, included studies use different sodium citrate administration method. Different concentration of solution and different type of the nozzle may have caused bias [23-25]. Secondly, dividing the participant with the degree of olfactory dysfunction such as anosmia and hyposmia or more detailed may be helpful since anosmia group is less likely to gain olfactory function improvement [26]. Thirdly, since sodium citrate is expected to have temporary effect of the sodium citrate, there has been no long-term study to analyze until now. Lastly, this meta-analysis included only three studies with a total of 267 patients. Further study with large, randomized, controlled clinical trial should be performed to provide more evidence on the efficacy of sodium citrate.
This study demonstrated that local application of sodium citrate in the patients with olfactory dysfunction did not improve olfactory function effectively but could cause frequent but trivial local adverse effect such as transient nasal or throat discomfort. Considering that calcium itself has multifunction on olfaction, not limited to negative feedback, we recommend that further well designed studies be needed to verify the clinical effectiveness of sodium citrate.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A1B07045421).
REFERENCES
1. Alt JA, Mace JC, Buniel MC, Soler ZM, Smith TL. Predictors of olfactory dysfunction in rhinosinusitis using the brief smell identification test. Laryngoscope. 2014; 124(7):E259–66.

2. Litvack JR, Fong K, Mace J, James KE, Smith TL. Predictors of olfactory dysfunction in patients with chronic rhinosinusitis. Laryngoscope. 2008; 118(12):2225–30.

3. Wang X, Zhu Y, Ni D, Lv W, Gao Z, Qi F. Intranasal application of glucocorticoid alleviates olfactory dysfunction in mice with allergic rhinitis. Exp Ther Med. 2017; 14(5):3971–8.

4. Katotomichelakis M, Simopoulos E, Tripsianis G, Prokopakis E, Danielides G, Velegrakis SG, et al. Improvement of olfactory function for quality of life recovery. Laryngoscope. 2013; 123(11):E10–6.

5. Holbrook EH, Leopold DA. An updated review of clinical olfaction. Curr Opin Otolaryngol Head Neck Surg. 2006; 14(1):23–8.

6. Park SW. Current trend of olfactory disorder management. Korean J Otorhinolaryngol Head Neck Surg. 2012; 55(5):272–7.

7. Panagiotopoulos G, Naxakis S, Papavasiliou A, Filipakis K, Papatheodorou G, Goumas P. Decreasing nasal mucus Ca++ improves hyposmia. Rhinology. 2005; 43(2):130–4.
8. Philpott CM, Erskine SE, Clark A, Leeper A, Salam M, Sharma R, et al. A randomised controlled trial of sodium citrate spray for nonconductive olfactory disorders. Clin Otolaryngol. 2017; 42(6):1295–302.

9. Whitcroft KL, Ezzat M, Cuevas M, Andrews P, Hummel T. The effect of intranasal sodium citrate on olfaction in post-infectious loss: Results from a prospective, placebo-controlled trial in 49 patients. Clin Otolaryngol. 2017; 42(3):557–63.

10. Whitcroft KL, Merkonidis C, Cuevas M, Haehner A, Philpott C, Hummel T. Intranasal sodium citrate solution improves olfaction in post-viral hyposmia. Rhinology. 2016; 54(4):368–74.

11. Menini A. Calcium signalling and regulation in olfactory neurons. Curr Opin Neurobiol. 1999; 9(4):419–26.

12. Leinders-Zufall T, Greer CA, Shepherd GM, Zufall F. Imaging odor-induced calcium transients in single olfactory cilia: Specificity of activation and role in transduction. J Neurosci. 1998; 18(15):5630–9.

13. Zufall F, Leinders-Zufall T, Greer CA. Amplification of odorinduced Ca(2+) transients by store-operated Ca(2+) release and its role in olfactory signal transduction. J Neurophysiol. 2000; 83(1):501–12.

14. Leinders-Zufall T, Ma M, Zufall F. Impaired odor adaptation in olfactory receptor neurons after inhibition of Ca2+/calmodulin kinase II. J Neurosci. 1999; 19(14):Rc19.

15. Kelliher KR, Ziesmann J, Munger SD, Reed RR, Zufall F. Importance of the CNGA4 channel gene for odor discrimination and adaptation in behaving mice. Proc Natl Acad Sci U S A. 2003; 100(7):4299–304.

16. Lötsch J, Reichmann H, Hummel T. Different odor tests contribute differently to the evaluation of olfactory loss. Chem Senses. 2008; 33(1):17–21.
17. Kobal G, Klimek L, Wolfensberger M, Gudziol H, Temmel A, Owen CM, et al. Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol. 2000; 257(4):205–11.

18. Doty RL, McKeown DA, Lee WW, Shaman P. A study of the test-retest reliability of ten olfactory tests. Chem Senses. 1995; 20(6):645–56.

19. Acion L, Peterson JJ, Temple S, Arndt S. Probabilistic index: An intuitive non-parametric approach to measuring the size of treatment effects. Stat Med. 2006; 25(4):591–602.

20. Saito S, Ayabe-Kanamura S, Takashima Y, Gotow N, Naito N, Nozawa T, et al. Development of a smell identification test using a novel stick-type odor presentation kit. Chem Senses. 2006; 31(4):379–91.

21. Klimmeck D, Mayer U, Ungerer N, Warnken U, Schnölzer M, Frings S, et al. Calcium-signaling networks in olfactory receptor neurons. Neuroscience. 2008; 151(3):901–12.

22. Choi R, Goldstein BJ. Olfactory epithelium: Cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig Otolaryngol. 2018; 3(1):35–42.

23. Chang H, Lee HJ, Mo JH, Lee CH, Kim JW. Clinical implication of the olfactory cleft in patients with chronic rhinosinusitis and olfactory loss. Arch Otolaryngol Head Neck Surg. 2009; 135(10):988–92.

Fig. 2.
Comparison between local application of sodium citrate and placebo in posttreatment score. Standardized mean difference of posttreatment score in identification (A) and threshold (B). TE: treatment ef-T fect, seTE: standard error of treatment effect, SMD: standardized mean difference, CI: confidence interval.
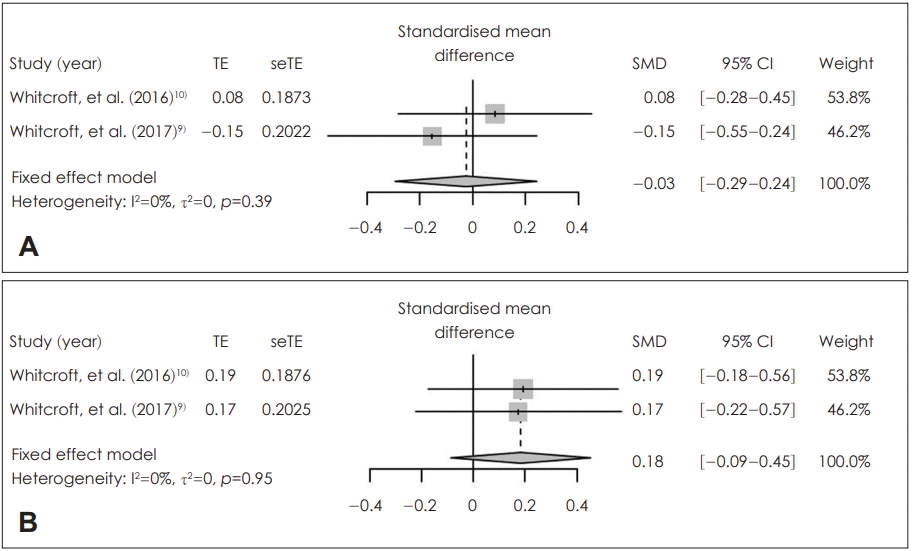
Fig. 3.
Comparison between local application of sodium citrate and placebo in pre-and post-treatment score change. Standardized mean difference of pre-and post-treatment score change in identification (A) and thresheold (B). TE: treatment effect, seTE: standard error of treatment effect, SMD: standardized mean difference, CI: confidence interval.
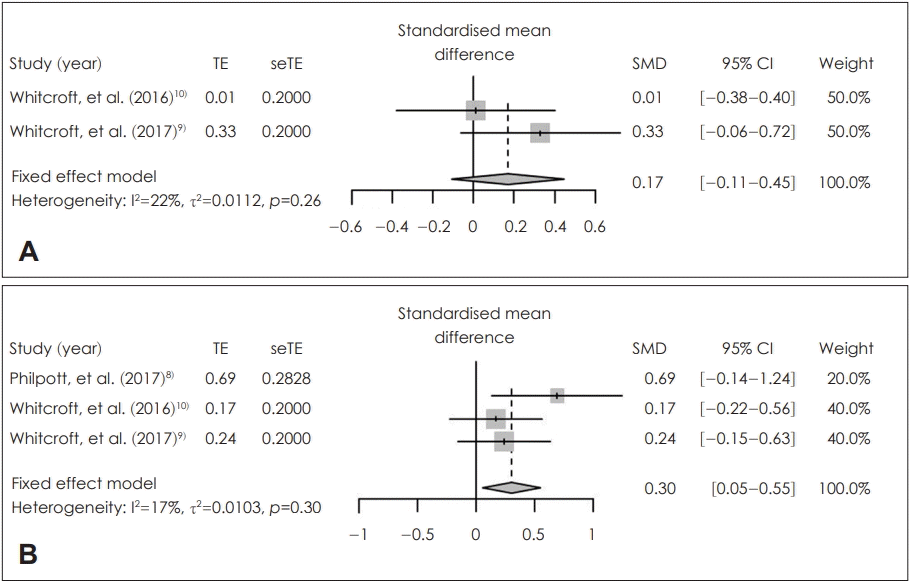
Fig. 4.
Effectiveness of local application of sodium citrate in treatment group. Standardized mean difference of pre-and post-treatment score change in identification (A) and threshold (B). TE: treatment effect, seTE: standard error of treatment effect, SMD: standardized mean difference, CI: confidence interval.
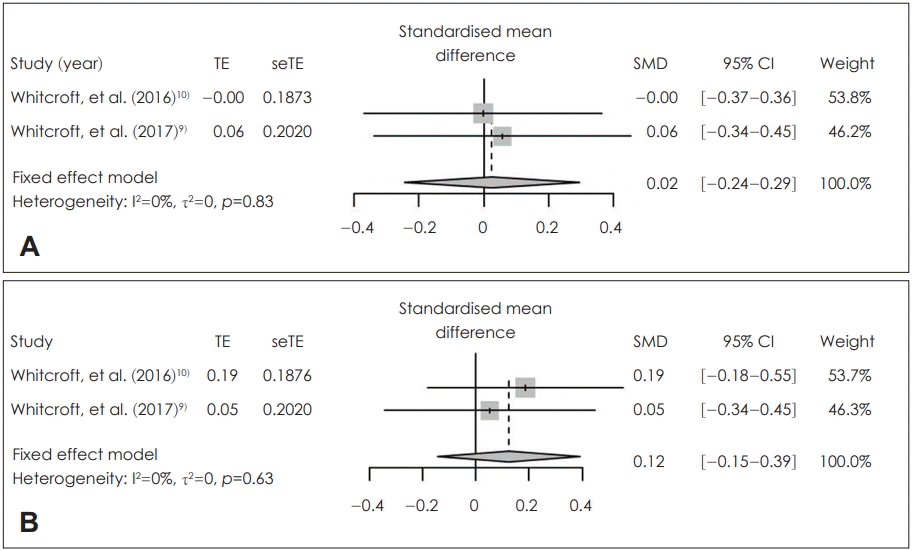
Table 1.
Summary of the studies included in the meta-analysis
| Study (year) | Sample size | Age (mean, range, or standard deviation)/ sex (ratio of male to female) | Study design | Comparison | Outcome measure analyzed | Risk of bias of randomized studies |
|---|---|---|---|---|---|---|
| Philpott, et al. (2017) [8] | 55 | 52.5 years (10.4 years)/13 males and 42 females | Randomized controlled | Sodium citrate (spray bottle and nozzle) vs. control (saline) | A series of threshold smell tests | Risk of bias (low risk) |
| Whitcroft, et al. (2016) [10] | 57 | 50.8 years (17.3 years)/30 males and 27 females | Randomized controlled | Sodium citrate (endoscopic application by physician) vs. control (saline) | “Sniffin' Sticks” test (identification test and threshold test) | Risk of bias (unclear risk) |
| Whitcroft, et al. (2017) [9] | 49 | 58.71 years (11.03 years)/11 males and 38 females | Randomized controlled | Sodium citrate (application with specific device by physician) vs. control (saline) | Sniffin' Sticks” test (identification test and threshold test) | Risk of bias (unclear risk) |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download