Abstract
Background and Objectives
Hearing loss is a common complication associated with Noonan syndrome (NS), and the level of hearing loss for NS patients with sensorineural loss ranged from normal to severe. Additional insights into the outcome of cochlear implantation (CI) in children with NS with or without comorbidities are needed.
Subjects and Method
In this study, five patients with NS, four with a mutation in PTPN11 and one who tested negative in mutation screening, diagnosed with the clinical scoring system and underwent CI at ages ranging from 16 to 50 months were retrospectively reviewed. Patients were evaluated for auditory perception (Categories of Auditory Performance), speech production (Korean Version of the Ling’s Stage), and language ability (Receptive and Expressive Vocabulary Test).
Results
In five of the children with NS, CI was performed without any complications. Three patients who received CI before 30 months of age showed outstanding outcomes. One patient who received CI at 50 months showed limited benefit. One patient who was diagnosed with developmental delay and cochlear nerve hypoplasia underwent CI at 28 months with poor outcome.Discussion Our report suggests that although the benefit may be influenced by comorbidities associated with NS or delay in hearing rehabilitation, profound hearing loss in patients with NS may be restored to normal levels in terms of auditory/speech perception and vocabulary/language development. The variable hearing outcomes also underscore the need for early screening and detection of profound hearing loss, and regular follow-up for hearing evaluation in patients with NS.
Noonan syndrome (NS) is inherited as an autosomal dominant disease with multiple abnormalities, and an estimated incidence of 1/1000 to 1/2500 live births [1,2]. NS is characterized by typical craniofacial dysmorphic features, short stature, and congenital heart defects and is associated with diverse clinical phenotypes in affected persons. NS is diagnosed via screening for gene mutations or using a clinical scoring system [3,4]. Nearly 50% of individuals with NS carry PTPN11 gene mutation. Other gene mutations including SOS1, RAF1, KRAS, NRAS, BRAF, and MAP2K1 have been implicated in NS etiology. However, 20% of clinically diagnosed patients lack biochemical markers associated with gene mutations.
Hearing loss is a common complication associated with NS. Otitis media is the most common cause of hearing loss [4]. Sharland, et al. [2] reported that the incidence of hearing loss in 146 patients with NS was 40%. In 3% of these patients, hearing loss was sensorineural and in the remaining patients, it involved a conductive hearing loss. In 2015, van Trier, et al. [5] reported the results of hearing evaluation in 34 patients with NS. Nine patients showed sensorineural hearing loss and three patients showed a progressive hearing loss. In addition, the hearing levels of individuals with sensorineural loss in NS ranged from normal hearing to severe hearing loss [5,6]. Inner ear abnormalities, including temporal bone and vestibular abnormalities have been described previously [7,8].
In the case of patients with severe-to-profound sensorineural hearing loss, cochlear implantation (CI) is indicated as a rehabilitation method. However, limited information is known concerning the outcome of CI in NS children with or without comorbidities. Therefore, we sought to report the outcomes of five children with NS who underwent a CI in their early ages.
We retrospectively reviewed a cohort of pediatric CI recipients in a tertiary referral center. Five NS patients were eligible subjects in this cohort. All patients were referred to an otorhinolaryngology clinic for a suspicious hearing loss in their first or second years of life after the newborn infant screening and were diagnosed with NS by pediatricians following screening for gene mutations or a clinical scoring system. Before assessing the hearing levels, full physical evaluations were performed and appropriate management including ventilation tube insertion was conducted if the patients showed evidence of otitis media. Therefore, a series of audiological tests were performed without any signs or symptoms of otitis media. Hearing loss was confirmed with a series of audiological evaluations, including Auditory Brainstem Response, Auditory Steady State Response, Oto Acoustic Emission, and visual reinforcement behavioral audiometry before using hearing aids bilaterally. Hearing amplification was used immediately after confirmation of the hearing loss. After the adequate use of hearing aids, we evaluated auditory/speech perception and speech production as pre-CI assessment within six months of CI. Temporal bone CT and MRI of the head were performed in all patients. To describe the age of the patients, the year, month or number of weeks were used.
This retrospective study was approved by Samsung Medical Center Institutional Review Board (SMC 2017-08-055-001).
Regular assessment post-CI included testing for auditory perception and speech. The auditory perception was assessed with Categories of Auditory Performance (CAP) scores. Speech production scale, the Korean Version of the Ling’s Stage (K-Ling) [9], was adapted to assess speech production. Both auditory perception and speech production were assessed by an experienced speech therapist.
To assess the vocabulary and language development, we used Receptive and Expressive Vocabulary Test (REVT) [10]. The REVT was developed to assess the receptive and expressive vocabulary abilities from infants to adults in the Korean population. To be eligible for evaluation, the individuals need to attain at least the 30-month level of appropriate language development. The results of REVT were displayed as an age equivalent according to the patient`s vocabulary development status.
Five NS patients including four with a PTPN11 mutation, and one testing negative in mutational screening and diagnosed with the clinical scoring system, were included. Age at the first CI ranged from 16 to 50 months. One patient (no. 3) underwent ventilation tube insertion bilateral for otitis media before the audiological tests. Two patients showed vestibulecochlear abnormalities in MRI, one with enlarged vestibular endolymphatic space and the other with hypoplastic cochlear nerve. One female patient (no. 4) was diagnosed with developmental delay at six months of age using the Denver developmental screening test. In all children, CI was successfully performed without any intraoperative complications despite various cardiac anomalies. Patients’ clinical characteristics were summarized in Table 1.
Results of initial and follow-up CAP scores were plotted for individual patients in Fig. 1. The first dots of each line indicate the results of preoperative evaluation. A single patient (no. 4) did not show any improvement after the CI and remained at CAP 1 score. Other patients showed gradual improvement in auditory perception after the CI.
Results of initial and follow-up K-Ling stages were plotted for individual patients in Fig. 2. The first dots of each line reveal preoperative test results. One patient (no. 4) remained in stage 1 after 4 years of CI. Patient no. 1 underwent CI at the age of 50 months and demonstrated improvement in speech production, and progressed to stage 4 (phonologic development) and stage 5 (phonetic development), which suggested that the patient uttered clear words with a satisfactory voice pattern, and used most vowels with good affricative sounds. Other patients showed gradual improvement in speech production and reached the maximum stage of phonetic and phonologic development in K-ling evaluation.
Results of REVT evaluations are plotted for individual patients in Fig. 3. Patient no. 4 did not show any improvement in vocabulary development. Patient no. 1 showed expressive (59 months in equivalent age) and receptive (77 months in equivalent age) development at age 14. Other patients showed gradual improvement in vocabulary development after CI.
It is well-known that early CI in infants with profound hearing loss is the key to achieving full potential, and reducing or eliminating the need for ‘catching up’ or learning spoken language at faster rates than normal, to achieve age-appropriate norms [11]. In this report, we described five patients with NS, four with a mutation associated with PTPN11 and one testing negative in mutation screening, and diagnosed with the clinical scoring system. The patients underwent CI at ages ranging from 16 to 50 months. All the patients started with hearing amplification immediately after the diagnosis except for one patient (no. 3), who underwent insertion of ventilation tube bilaterally due to otitis media and delayed confirmation of hearing loss. All the patients were evaluated for performance outcomes regarding auditory perception (CAP), speech production (K-Ling’s stage), and language ability (REVT). Three patients who received CI before 30 months of age showed outstanding outcomes. One patient who received CI at 50 months showed limited benefit. However, his auditory perception and language ability were gradually improved with proper rehabilitation and speech therapy. The patient with developmental delay and hypoplastic cochlear nerve in MRI scan showed limited development despite early implantation. Developmental delay and cochlear nerve hypoplasia are factors associated with poor CI outcomes in children [12,13]. Other patients diagnosed with early CI in our study received appropriate benefit and showed development in language and auditory perception following CI. Children with NS and diagnosed with profound hearing loss represent candidates for hearing rehabilitation following CI. Early intervention with CI in NS contributes to development of spoken language and intelligible speech in line with their peer groups.
Studies reported the outcomes of CI in eight patients carrying a PTPN11 mutation and diagnosed with NS or NS with multiple lentigines (NSML) [14-16]. In the study of van Nierop, et al. [14] reporting the outcome of five children with NS or NSML, all the patients who received CI showed audiological improvement. However, they reported a limited benefit of CI in terms of language development, due to patients’ intellectual disability and severe hearing loss during the first few years of life. We provide additional insights into the outcome of CI in children with NS with or without comorbidities. We showed that children with NS without intellectual comorbidities achieved not only audiological improvement but also developed language skills.
In a few patients with NS, a histopathologic analysis revealed hearing loss associated with inner ear abnormalities including a reduced number of spiral ganglia, enlarged lateral semicircular canal, and dislocated endolymphatic sac and vestibular aqueduct [17]. The structural and vestibular abnormalities associated with the inner ear may manifest as sensorineural hearing loss in NS. Sensorineural hearing loss in NS ranged from normal or mild high-frequency hearing loss to a profound (uni- or bilateral) hearing loss [5,6]. The prevalence of hearing loss and possible delay in speech associated with NS were documented in previous reports. Therefore, individuals suspected with NS are required to undergo hearing evaluation at an early age. A careful follow-up from early childhood throughout life is recommended. Hearing aids and other aural rehabilitation should be used by children with more than a mild hearing loss as early as possible. Individuals with NS diagnosed with profound hearing loss require appropriate rehabilitation using CI.
Interestingly, this report included one patient (no. 4) who was diagnosed with profound hearing loss and underwent CI at age 28 months. This is the first patient with NS who received the CI without a diagnosed ,PTPN11 mutation. She tested negative in gene mutation tests and was therefore, diagnosed with a clinical scoring system. Incomplete data are available regarding the deaf patient without PTPN11 gene mutation. Therefore, patients with NS manifest a potentially diverse range of diagnostic results associated with hearing loss.
In summary, our report suggests that although the benefits of treatment and rehabilitation may be affected by comorbidities associated with NS or delayed hearing, patients with NS manifesting profound hearing loss can regain their linguistic abilities on par with normal peers. In addition, the variable expression of hearing levels in patients with NS underscores the need for early screening and evaluation of profound hearing loss, with regular follow-up.
REFERENCES
1. Noonan JA. Hypertelorism with Turner phenotype. A new syndrome with associated congenital heart disease. Am J Dis Child. 1968; 116(4):373–80.
2. Sharland M, Burch M, McKenna WM, Paton MA. A clinical study of Noonan syndrome. Arch Dis Child. 1992; 67(2):178–83.

3. van der Burgt I, Berends E, Lommen E, van Beersum S, Hamel B, Mariman E. Clinical and molecular studies in a large Dutch family with Noonan syndrome. Am J Med Genet. 1994; 53(2):187–91.

5. van Trier DC, van Nierop J, Draaisma JM, van der Burgt I, Kunst H, Croonen EA, et al. External ear anomalies and hearing impairment in Noonan syndrome. Int J Pediatr Otorhinolaryngol. 2015; 79(6):874–8.

6. Qiu WW, Yin SS, Stucker FJ, et al. Audiologic manifestations of Noonan syndrome. Otolaryngol Head Neck Surg. 1998; 118(3 Pt 1):319–23.

7. Naficy S, Shepard NT, Telian SA. Multiple temporal bone anomalies associated with Noonan syndrome. Otolaryngol Head Neck Surg. 1997; 116(2):265–7.

8. Foster CA, Dyhrkopp PJ. Noonan’s syndrome with sensorineural hearing loss and vestibular abnormalities. Otolaryngol Head Neck Surg. 1998; 119(5):508–11.

9. Moon IJ, Kim EY, Chu H, Chung WH, Cho YS, Hong SH. A new measurement tool for speech development based on Ling’s stages of speech acquisition in pediatric cochlear implant recipients. Int J Pediatr Otorhinolaryngol. 2011; 75(4):495–9.

10. Kim YT, Hong GH, Kim KH. Content and reliability analyses of the receptive and expressive vocabulary test (REVT). Commun Sci Disord. 2009; 14(1):34–45.
11. Driver S, Jiang D. Paediatric cochlear implantation factors that affect outcomes. Eur J Paediatr Neurol. 2017; 21(1):104–8.

12. Birman CS, Elliott EJ, Gibson WP. Pediatric cochlear implants: additional disabilities prevalence, risk factors, and effect on language outcomes. Otol Neurotol. 2012; 33(8):1347–52.
13. Kang WS, Lee JH, Lee HN, Lee KS. Cochlear implantations in young children with cochlear nerve deficiency diagnosed by MRI. Otolaryngol Head Neck Surg. 2010; 143(1):101–8.

14. van Nierop JWI, van Trier DC, van der Burgt I, Draaisma JMT, Mylanus EAM, Snik AF, et al. Cochlear implantation and clinical features in patients with Noonan syndrome and Noonan syndrome with multiple lentigines caused by a mutation in PTPN11. Int J Pediatr Otorhinolaryngol. 2017; 97:228–34.

15. Chu HS, Chung HS, Ko MH, Kim HJ, Ki CS, Chung WH, et al. Syndromic Hearing Loss in Association with PTPN11-related disorder: the experience of cochlear implantation in a child with LEOPARD syndrome. Clin Exp Otorhinolaryngol. 2013; 6(2):99–102.
Fig. 1.
Plots of auditory perception outcomes in individual subjects. Each color and connectedline denote a single patient. The x-axis represents the chronologic age and y-axis represents scores assessed with CAP scores. CAP: Categories of Auditory Performance.
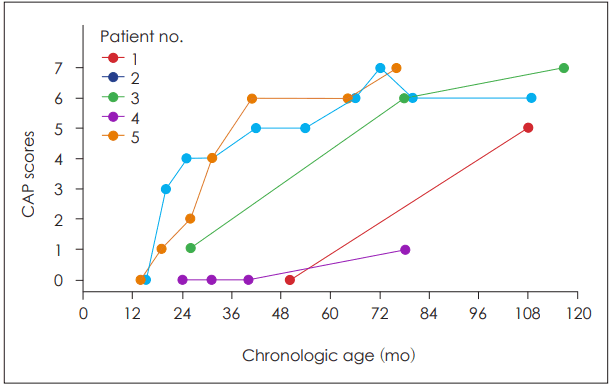
Fig. 2.
Plots of speech perception outcomes involving individual subjects. Phonetic development (A) and phonologic development (B). Each color and connected-line denote a single patient. The x-axis represents the chronologic age and y-axis represents scores assessed with K-Ling’s stages. K-Ling’s stages: Korean Version of the Ling’s Stage.
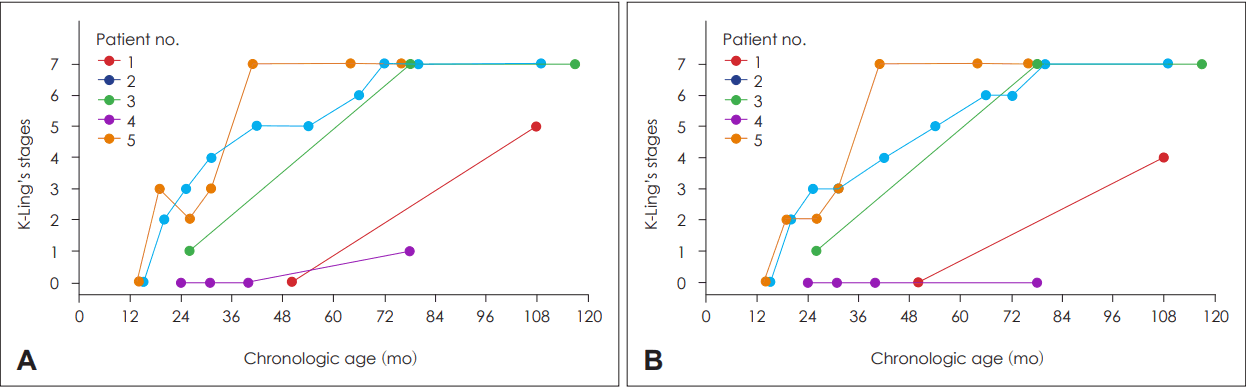
Fig. 3.
Plots of vocabulary and language development. Expressive domain (A) and receptive domain (B). Each color and connectedline denote a single patient. The x-axis represents the chronologic age and y-axis represents appropriate equivalent age with Receptive and Expressive Vocabulary Test.
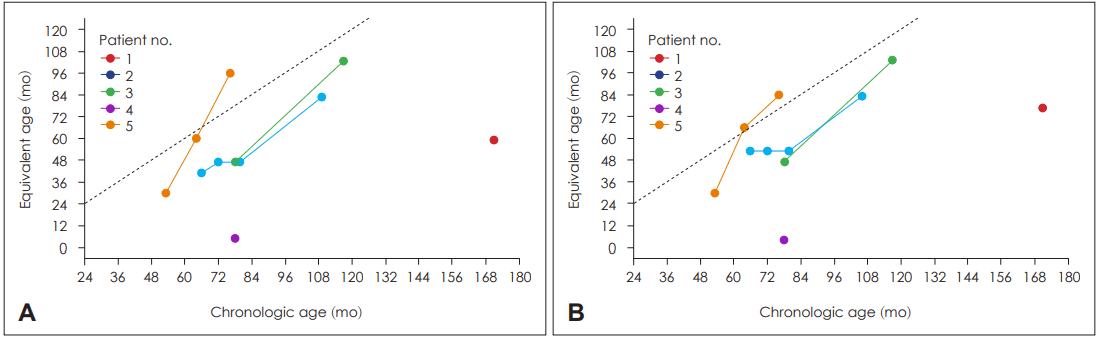
Table 1.
Clinical characteristics of the study patients




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download