Abstract
Background and Objectives
Several studies have reported the benefits of olfactory training (OT) for patients with olfactory dysfunction. However, training odorants should be customized according to the characteristics of individual patients. The aim of this study was to evaluate the effects of OT using odorants familiar to Korean patients with post-upper respiratory infection (URI) olfactory dysfunction.
Subjects and Methods
We prospectively evaluated patients with post-URI olfactory loss. We carried out OT over a period of 6 months using four odorants: pine, cinnamon, lemon, and peppermint. Olfaction was tested before and at 1, 3, and 6 months after training initiation using the following olfactory tests; Butanol threshold test (BTT), Cross-Cultural Smell Identification Test (CCSIT) and Visual Analog Scale (VAS), Nasal Obstruction Symptom Evaluation (NOSE) scale, Sino-Nasal Outcome Test (SNOT-20), and Beck Depression Inventory (BDI).
Results
Of the 88 patients who initially enrolled, 82 completed the BTT, CCSIT, VAS, NOSE, SNOT-20, and BDI. In total, 10 Korean patients were included in our analysis, nine of whom showed an improvement in olfaction after OT. All patients reported changes in olfaction and the perception of smells over the duration of OT. Some patients reported an increased sense of smell after OT, although he could not distinguish different smells.
Conclusion
OT using odorants specifically selected for Korean patients resulted in improved olfactory function, as indicated by the BTT and CCSIT scores. The findings of the present study suggest that customization of odorants to fit the characteristics of patients, including ethnicity, environment, and race, among others, increases the effectiveness of OT.
Approximately 5% of the general population are considered to be anosmic, and an additional 15% are considered to be hyposmic [1]. Upper respiratory infections (URIs) are among the most frequent causes of olfactory dysfunction [2-4]. To date, there is no validated pharmacotherapy for post-URI olfactory dysfunction, although attempts have been made to establish treatment modalities through trials involving systemic and topical steroids [5,6]. Furthermore, repeated short-term exposure to odorants so-called olfactory training (OT), vitamin B [7], caroverine [8], alpha-lipoic acid [9], minocycline [10], and acupuncture [11] appears to be effective in improving olfaction.
Hummel, et al. [12] determined that OT for >12 weeks increased olfactory function in 28% of participants. They used eucalyptus, clove, lemon, and rose as odorants in their study. In a recent randomized study, the same authors extended the duration of OT from 12 to 18 weeks, and they obtained results similar to those obtained in the original study [1].
However, in Korea, patients cannot recognize the odors of eucalyptus and clove, considering that Asians, particularly Koreans, are not familiar with these substances. In fact, some Koreans have never seen or smelt these plants. Therefore, the results of OT using plants grown in the West, such as clove, may not be as good as those of OT using Korean plants as odorants for Korean patients with olfactory dysfunction. Based on the hypothesis that the use of familiar odors may improve the results of OT, we designed the present study using Korean odorants for OT in a cohort of Korean patients with olfactory dysfunction.
All participants in the present study were self-referrals from an outside institution to the Department of Otorhinolaryngology. This study was performed in accordance with the principles of the Declaration of Helsinki, and the Institutional Review Board and ethics committee at Catholic Medical Center approved the study protocol. The experimental design was approved by the ethics committee of the medical faculty at Uijeonbu St. Mary’s hospital, and written informed consent was obtained from all patients. A total of 88 patients with olfactory dysfunction were initially enrolled (49 women, 39 men; mean age, 53.5±15.5 years; range, 19-81 years). All patients underwent thorough examinations, including endoscopy and CT of the nasal cavities, conducted by an experienced otorhinolaryngologist. The inclusion criteria were as follows: olfactory dysfunction and a recent history of URI. The exclusion criteria were as follows: pregnancy, chronic rhinosinusitis, nasal polyposis, allergic or idiopathic rhinitis, post-traumatic olfactory loss, other acute or chronic nasal, malignant tumors and/or a history of oncology therapies (radiation, chemotherapy), and a history of surgery in the nose or paranasal sinuses (IRB No. UC15RISI0061).
At the first visit (baseline), a thorough medical history was recorded using a standardized case report form. In addition, a systematic otorhinolaryngological examination, including endoscopy of the olfactory cleft, was performed to exclude nasal pathologies. The diagnosis of olfactory dysfunction was classified as post-URI depending on the clinical findings and past medical history. Olfactory threshold testing was performed using the Butanol threshold test (BTT). And olfactory identification testing was performed using the Cross-Cultural Smell Identification Test (CCSIT). The clinical evaluation of nasal and psychological function was based on the composite Visual Analog Scale (VAS), Nasal Obstruction Symptom Evaluation (NOSE) scale, Sino-Nasal Outcome Test (SNOT-20), and Beck Depression Inventory (BDI) scores. Participants diagnosed with olfactory dysfunction were prescribed oral steroids 20 mg for 1 week and 10 mg for 1 week. On completion of the steroid course, we initiated OT using Korean odorants; we initially treated patients using steroids because it is a known effective treatment modality [5]. All participants were reassessed using endoscopy, BTT, and CCSIT at 1, 3, and 6 months after OT initiation. In addition, patients were evaluated using the VAS, NOSE scale, SNOT-20, and BDI before, and at 1, 3, and 6 months after OT.
OT was performed over a period of 6 months. The patients were instructed to expose themselves twice daily to four Korean odorants: pine, cinnamon, lemon, and peppermint. The lemon and cinnamon odorants were chosen as representatives of the four odor categories included in the Korean version of the Sniffn’ Sticks (KVSS) test [13]. Peppermint was chosen on the basis of the KVSS and other studies assessing the olfactory system [14]. Finally, pine was chosen was because it is one of the most common trees in South Korea.
The patients received four plastic tubes (total volume, 10 mL) with one of the four odorants in each. All tubes were labeled with the odorant name. Patients were advised to sniff the odorants in the morning and evening for approximately 10 seconds each, with a rest period of 30 seconds between each odorant to prevent olfactory fatigue [15]. All patients were instructed to maintain a diary to keep their attention focused on training. After the initial visit before OT, patients were followed up at 1, 3, and 6 months after OT. At every visit, patients were educated and encouraged with regard to OT. In addition, they were questioned about subjective changes in their olfactory function and were advised to maintain compliance with the training protocol.
Among the 88 patients who were initially enrolled, 82 completed the BTT, CCSIT, VAS, NOSE, SNOT-20, and BDI. Six patients were exclude due to uncompleted the olfactory test or questionares. The mean baseline BDI score was 6.7 for these patients, which indicated mild depression. The mean baseline VAS scores for nasal obstruction, sneezing, snoring, headache, facial pain, and olfactory dysfunction were 2.4, 3.0, 3.0, 2.1, 1.18, and 6.7, respectively.
After the first visit, 56 patients were lost to follow-up, and 26 patients remained at the 3-month follow-up. Of these 26 patients, 16 patients did not continue their OT. The remaining 10 patients were included in our study. All 10 patients reported an impaired sense of smell and taste. Nine of the 10 patients exhibited improved olfaction after OT (Fig. 1). BTT and CCSIT scores increased for four patients (patients 1, 3, 5, and 10) after 3 months of OT, while a subjective improvement in olfactory function was observed in the remaining five patients (patients 2, 4, 6, 7, and 8) at the 3- or 6-month follow-up (Table 1). Patient 1 showed a 3-point increase in BTT score and no change in CCSIT score. Patient 3 showed a 4-point increase in BTT score and a 1-point increase in CCSIT score. Patient 5 showed a 1-point increase in BTT score and a 1-point increase in CCSIT score. Patient 10 showed an 8-point increase in BTT score and a 2-point increase in CCSIT score. One patient (patient 9) tested her olfaction after 3 months and exhibited no subjective improvements. In fact, her BTT score decreased by 3 points. Nine patients reported changes in olfaction and the perception of smells over the duration of OT. In detail, patient 2 reported alleviation of his depressive state after 3 months of OT, and that his perception of shampoo smell had changed after 6 months of OT. Patient 3 reported an increased sense of smell after 3 months of OT, although he could not distinguish different smells at that time. Patient 4 reported that he could only smell briefly after 3 months of OT. Patient 5 reported that his perception of a toilet smell was wrong after 3 months of OT, but that this was rectified after 6 months of OT. Patient 10 perceived the smell of cucumber as a fishy smell after 3 months of OT. We were unable to fully analyze the VAS, NOSE, SNOT-20, and BDI scores after 3 and 6 months because the patients did not completely answer all of the questions.
In the present study, we evaluated the effects of OT using Korean odorants in Korean patients with post-URI olfactory dysfunction and found that most of the included patients exhibited an improvement in their olfactory function after OT.
Recent studies have shown that olfactory disorders occur at a much higher rate than previously assumed [12]. Hendriks [16] reported a 35% recovery rate over approximately 12 months. Reden, et al. [17] reported that 32% of patients exhibited significant improvement after a mean interval of 14 months. Heilmann, et al. [18] showed that 35% of patients exhibited a marked increase in olfactory function over an average period of four months. However, regardless of how long it may take to olfactory recovery, olfactory loss has been shown to have a severe impact on the quality of life in some patients [19]. Despite the fact that numerous studies have shown a regenerative ability for olfactory receptor neurons, few treatment options have been proven effective for post-URI dysosmia; One of the most common causes of dysosmia [20,21]. Numerous therapeutic strategies have been proposed, such as the use of strychnine, zinc, theophylline, lipoic acid, and caroverine [3,8,9,22-27]. Although the effectiveness of most of these regimens is far from clear, the usefulness of corticosteroids in sensorineural dysfunction-related olfactory loss is established. This is supported by the observation that systemic steroids are helpful in sensorineural dysfunction-related olfactory loss. However, some patients cannot use oral steroids because of systemic complications. In particular, the long-term use of steroids can result in more severe complications such as stroke, heart disease, and diabetes [5].
Of late, OT has been shown to improve olfactory function in humans [28-30]. A recent systematic review and meta-analysis suggested that OT may be an effective intervention for patients with olfactory dysfunction [31]. Most other studies have reported positive outcomes of OT with regard to olfaction, without significant adverse effects.
Repeated exposure of healthy individuals to odorants was shown to significantly increase olfactory sensitivity, improve the recovery of patients with post-viral olfactory loss [32]. The results of the OT meta-analysis were consistent with the reported results of individual studies, primarily with regard to an improvement in the Threshold-Discrimination-Identification (TDI) score.
In the present study, we derived four important and unique findings compared with the findings of recent studies. First, nine of 10 patients who received OT for a long duration showed an improvement in olfaction. This rate is higher than that in other studies [32], probably because our Korean odorants were more effective for Korean patients, compared to the scents of eucalyptus, clove, lemon, and rose, which were designed for Western patients [1,12]. A biased odor selection maight be a limitation. The four odorants chosen by Hummel, et al. [12] were within the four odor categories claimed by Henning and Der [33] in his workon the ‘odor prism.’ Among our odor selection (pine, cinnamon, lemon, and peppermint), flowery axis is lacking. Also, pine and lemmon are same axis. However, we selected those odors because the use of familiar smells in OT might be more effective than that of unfamiliar smells and could aid patients in better identification.
Second, the patients reported changes in olfaction and the perception of smells during the duration of OT. These findings may be a result of differences in the mechanisms underlying an improvement in the olfactory threshold and olfactory identification, although the level at which these changes occurred, e.g., olfactory receptors, synapses, or central nerves, remains unclear.
Three of the 10 patients showed an improvement of >3 points in their BTT score, and the improvement in the CCSIT score came later than that in the BTT score. These findings suggest that the olfactory threshold may improve faster than olfactory identification when olfactory nerves are regenerated. In other words, the progress of regeneration probably differs between the olfactory threshold and olfactory identification [34].
The olfactory system exhibits extraordinary plasticity because of mechanisms that have been extensively investigated at the cognitive as well as the cellular level [35,36]. The mechanisms of neural plasticity in the olfactory system are interesting, because olfactory loss is among the first symptoms of neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease [37,38]. In addition, there is a loss of, or at least a decrease in, olfactory function in many neurological conditions [39]. Therefore, neural plasticity with regard to olfactory loss may have widespread implications for brain function. However, the mechanisms underlying the neural plasticity of the olfactory system are still under investigation [38]. It is known that sensory loss often entails functional and structural modifications in the central nervous system [40,41]. An understanding of the extent and mechanisms of plasticity in the olfactory system might provide insight into the brain mechanisms underlying recovery and reorganization [38]. These findings suggest that OT may induce extensive reorganizational processes in more than just the olfactory areas, i.e., OT may strengthen cognitive function beyond olfactory perception. In addition to the central effects of OT manifesting as network changes, there was a statistically significant improvement in the odor detection threshold in other studies [42,43].
Third, our results indicated the need for changes in conventional olfactory testing methods. We found that two patients complained of serious olfactory dysfunction that caused them great inconvenience after URI. However, their BTT and CCSIT scores were 8 and 9, which fall within the normal range. On the other hand, patient 2 had BTT and CCSIT scores of 5, which indicates abnormal olfactory function, although he led a comfortable life without any problems. This suggests that the findings of tests indicating normosmia and hyposmia may not correspond to the clinical findings of olfactory dysfunction. Accordingly, more appropriate tests and criteria are desirable for grading olfactory dysfunction.
Fourth, 7 patients who experienced olfactory dysfunction for 2-3 months after URI showed no improvement after oral steroid therapy in the present study. However, after 3 months of OT, all seven patients exhibited improved olfaction, as indicated by their BTT and CCSIT scores and a subjective improvement in symptoms. This demonstrates the effectiveness of OT. However, we do not believe that OT can serve as a replacement for oral steroid therapy, because according to our experience and the results of other studies, oral steroids are effective in the recovery of olfaction [5]. However, it was reported that two-thirds of patients who received local corticosteroids experienced little or no improvement in olfactory function, particular with regard to long-term changes [5]. We believe that an effective and definite alternative is required for the management of olfactory dysfunction, and OT can be that alternative treatment as assistant to steroid therapy.
The present study was limited in that it had a small sample size and high rate of drop-out. However, we treated 13 anosmic patients with oral steroid without OT in our clinic. Only one patient recovered his olfaction. And a single doctor followed up all patients from diagnosis through treatment over a 2-year period, and also analyzed all their data. Therefore, we believe that the data is accurate and robust. Moreover, all patients were consistently educated throughout the study period. This is necessary to encourage patients to persist with OT, and continuous interaction between doctors and patients, as well as appropriate patient education are important for successful OT. Maintenance of a diary, for example, may aid in increasing long-term compliance with training.
Future studies should investigate the long-term effects of OT to shed light on its impact on higher olfactory function, which may require a longer training period for recovery, considering the recovery of basic sensory perception is required for the regeneration of higher function. In addition, future studies can present a detailed description of changes in the brain during OT using functional MRI. A study has already documented olfactory bulb assessments using MRI; the results showed that the olfactory bulb volume was significantly larger after OT [44]. However, the sample size was small in that study. Mammalian models can also be used for such experiments, although it is difficult to create models of olfactory dysfunction and correctly measure olfaction in such models. However, this is an area of research that we plan to focus on. In addition, we are hoping to design tools for the direct visualization of nerve regeneration in the near future.
In conclusion, OT is a cost-effective and noninvasive treatment option for olfactory dysfunction. However, we cannot conclude that it is suitable as a sole treatment. Instead, the findings of our study and previous studies suggest that OT is an effective long-term ancillary treatment for oral steroid therapy, without the complications associated with steroid therapy. Furthermore, odorants should be customized according to the individual patient’s characteristics, including race, environment, and residence. Odorants familiar to the patient should be used for effective OT, and the development of such odorants is an essential clinical task.
ACKNOWLEDGMENTS
The author wish to acknowledge the financial support of The Catholic University of Korea Uijeongbu St. Mary’s Hospital Clinical Research Laboratory Foundation made in the program year of 2017 and 2018.
REFERENCES
1. Damm M, Pikart LK, Reimann H, Burkert S, Göktas Ö, Haxel B, et al. Olfactory training is helpful in ostinfectious olfactory loss: a randomized controlled multicenter study. Laryngoscope. 2014; 124(4):826–31.
3. Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, et al. Smell and taste disorders, a study of 50 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991; 117(5):519–28.
4. Damm M, Temmel A, Welge-Lüssen A, Eckel HE, Kreft MP, Klussmann JP, et al. Olfactory dysfunctions. Epidemiology and therapy in Germany, Austria and Switzerland. HNO. 2004; 52(2):112–20.
5. Heilmann S, Huttenbrink KB, Hummel T. Local and systemic administration of corticosteroids in the treatment of olfactory loss. Am J Rhinol. 2004; 18(1):29–33.

6. Stenner M, Vent J, Huttenbrink KB, Hummel T, Damm M. Topical therapy in anosmia: relevance of steroid-responsiveness. Laryngoscope. 2008; 118(9):1681–6.

7. Hummel T, Landis BN, Hüttenbrink KB. Smell and taste disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2011; 10:Doc04.
8. Quint C, Temmel AF, Hummel T, Ehrenberger K. The quinoxaline derivative caroverine in the treatment of sensorineural smell disorders: a proof-of-concept study. Acta Otolaryngol. 2002; 122(8):877–81.

9. Hummel T, Heilmann S, Hüttenbriuk KB. Lipoic acid in the treatment of smell dysfunction following viral infection of the upper respiratory tract. Laryngoscope. 2002; 112(11):2076–80.

10. Reden J, Herting B, Lill K, Kern R, Hummel T. Treatment of postinfectious olfactory disorders with minocycline: a double-blind, placebo-controlled study. Laryngoscope. 2011; 121(3):679–82.

11. Vent J, Wang DW, Damm M. Effects of traditional Chinese acupuncture in post-viral olfactory dysfunction. Otolaryngol Head Neck Surg. 2010; 142(4):505–9.

12. Hummel T, Rissom K, Reden J, Hähner A, Weidenbecher M, Hüttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009; 119(3):496–9.

13. Cho JH, Jeong YS, Lee YJ, Hong SC, Yoon JH, Kim JK. The Korean version of the Sniffin’ stick (KVSS) test and its validity in comparison with the cross-cultural smell identification test (CC-SIT). Auris Nasus Larynx. 2009; 36(3):280–6.

14. Johnson AJ. Cognitive facilitation following intentional odor exposure. Sensors (Basel). 2011; 11(5):5469–88.

15. Poellinger A, Thomas R, Lio P, Lee A, Makris N, Rosen BR, et al. Activation and habituation in olfaction--an fMRI study. Neuroimage. 2001; 13(4):547–60.

16. Hendriks AP. Olfactory dysfunction. Rhinology. 1988; 26(4):229–51.
17. Reden J, Mueller A, Mueller C, Konstantinidis I, Frasnelli J, Landis BN, et al. Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 2006; 132(3):265–9.

18. Heilmann S, Just T, Göktas O, Hauswald B, Hüttenbrink KB, Hummel T. Effects of systemic or topical administration of corticosteroids and vitamin B in patients with olfactory loss. Laryngorhinootologie. 2004; 83(11):729–34.
19. Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol. 2005; 125(2):116–21.

20. Landis B, Giger R, Morabia A, Ringgenberg R, Schilling B, Ricchetti A, et al. Olfaction: an epidemiological study on 1046 subjects. Chem Senses. 2003; 28:E33.
21. Schwob JE, Youngentob SL, Ring G, Iwema CL, Mezza RC. Reinnervation of the rat olfactory bulb after methyl bromide-induced lesion: timing and extent of reinnervation. J Comp Neurol. 1999; 412(3):439–57.

22. Seiden AM, Duncan HJ. The diagnosis of a conductive olfactory loss. Laryngoscope. 2001; 111(1):9–14.

23. Stoll AL, Oepen G. Zinc salts for the treatment of olfactory and gustatory symptoms in psychiatric patients: a case series. J Clin Psychiatry. 1994; 55(7):309–11.
25. Jafek BW, Moran DT, Eller PM, Rowley JC 3rd, Jafek TB. Steroid-dependent anosmia. Arch Otolaryngol Head Neck Surg. 1987; 113(5):547–9.

26. Hirsch AR, Dougherty DD, Aranda JG, Vanderbilt JG, Weclaw GC. Medications for olfactory loss: pilot studies. J Neurol Orth Med S. 1996; 17(2):109–14.
27. Levy LM, Henkin RI, Lin CS, Hutter A, Schellinger D. Increased brain activation in response to odors in patients with hyposmia after theophylline treatment demonstrated by fMRI. J Comput Assist Tomogr. 1998; 22(5):760–70.

28. Cain WS, Stevens JC, Nickou CM, Giles A, Johnston I, Garcia-Medina MR. Life-span development of odor identification, learning, and olfactory sensitivity. Perception. 1995; 24(12):1457–72.

29. Engen T, Bosack TN. Facilitation in olfactory detection. J Comp Physiol Psychol. 1969; 68(3):320–6.

30. Livermore A, Laing DG. Influence of training and experience on the perception of multicomponent odor mixtures. J Exp Psychol Hum Percept Perform. 1996; 22(2):267–77.

31. Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2016; 6(3):299–307.

32. Schriever VA, Lehmann S, Prange J, Hummel T. Preventing olfactory deterioration: olfactory training may be of help in older people. J Am Geriatr Soc. 2014; 62(2):384–6.

33. Henning H, Der geruch . Leipzig: Johann Ambrosius Barth. 1916.
34. Kim DH, Kim SW, Hwang SH, Kim BG, Kang JM, Cho JH, et al. Prognosis of olfactory dysfunction according to etiology and timing of treatment. Otolaryngol Head Neck Surg. 2017; 156(2):371–7.

35. Mainland JD, Bremner EA, Young N, Johnson BN, Khan RM, Bensafi M, et al. Olfactory plasticity: one nostril knows what the other learns. Nature. 2002; 419(6909):802.
36. Wilson DA, Best AR, Sullivan RM. Plasticity in the olfactory system: lessons for the neurobiology of memory. Neuroscientist. 2004; 10(6):513–24.

37. Doty RL. Olfaction in Parkinsons disease and related disorders. Neurobiol Dis. 2012; 46(3):527–52.

38. Marine N, Boriana A. Olfactory markers of depression and Alzheimer’s disease. Neurosci Biobehav Rev. 2014; 45:262–70.
39. Doty RL. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol. 2008; 63(1):7–15.

40. Qin W, Xuan Y, Liu Y, Jiang T, Yu C. Functional connectivity density in congenitally and late blind subjects. Cereb Cortex. 2015; 25(9):2507–16.

41. Bola M, Gall C, Moewes C, Fedorov A, Hinrichs H, Sabel BA. Brain functional connectivity network breakdown and restoration in blindness. Neurology. 2014; 83(6):542–51.

42. Larsson M, Semb H, Winblad B, Amberla K, Wahlund LO, Bäckman L. Odor identification in normal aging and early Alzheimer’s disease: effects of retrieval support. Neuropsychology. 1999; 13(1):47–53.

Fig. 1.
Flow chart of olfactory training for olfactory disturbance patients. BTT: Butanol threshold test, CCSIT: Cross-Cultural Smell Identification Test, URI: upper respiratory infection.
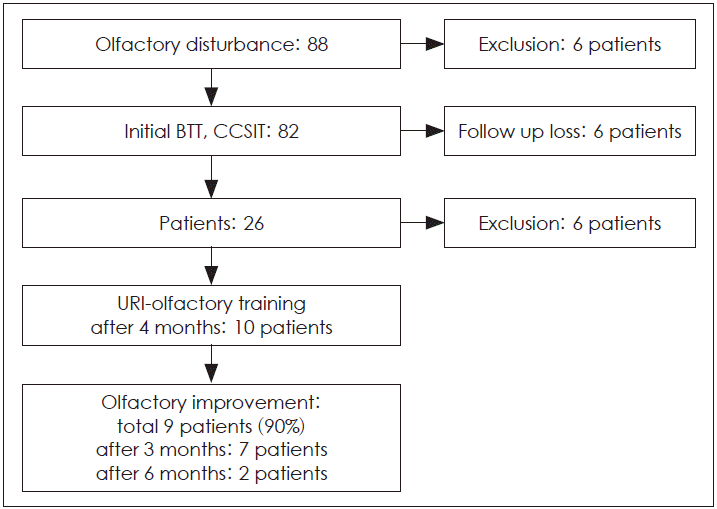
Table 1.
Clinical features of ten patients with olfactory disturbance after upper respiratory infection




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download