In the
Korean Journal of Anesthesiology, four case reports of SLKT were identified. In 2002, Lee et al. [
4] reported a case of SLKT (living liver and kidney donor) in a 45-year-old man receiving preoperative hemodialysis. Authors discussed the case with transplant surgeons and decided not to use ioRRT because of its unfamiliarity. Instead, they decided to implant the kidney graft earlier and the liver graft later, to ease the intraoperative management of renal dysfunction. Liver grafting is usually implanted earlier than kidney grafting to avoid kidney graft hypoperfusion during the liver reperfusion period, and to shorten the ischemia time of liver grafting, which is known to be more more limited than that of the kidney grafting. However, the authors in that case concluded that in the living donor LT, ischemia time could be shortened by timing the operation of the donor to that of the recipient. The implanted kidney graft functioned well during whole operation, including in the liver reperfusion period, and anesthesia was maintained successfully.
In 2009, Park et al. [
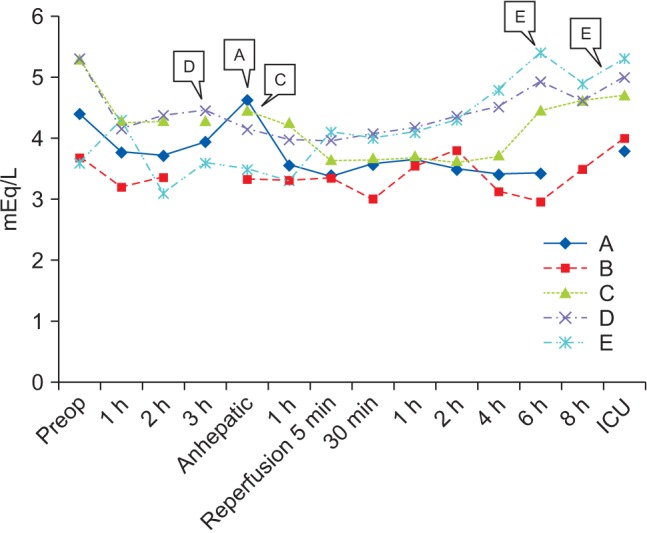
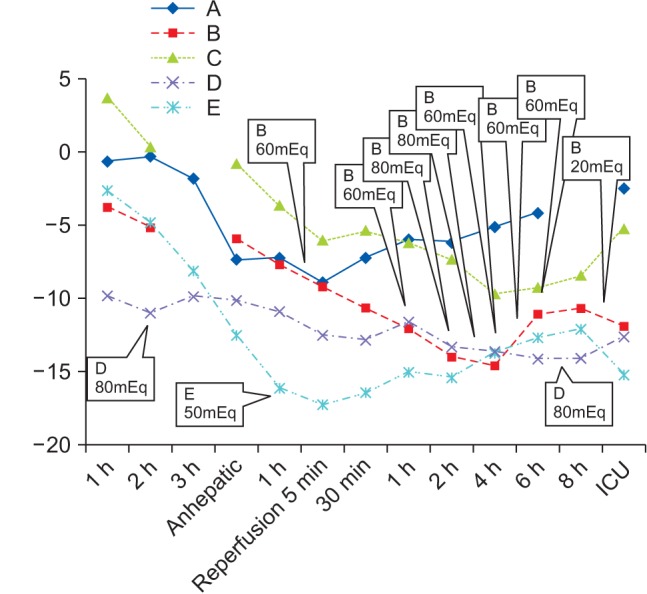
5] reported a case of SLKT (deceased liver and kidney donor) in a 13-year-old child receiving preoperative peritoneal dialysis. Authors found it difficult to place an additional large caliber central line in a child, and so did not use ioRRT. Instead, they used non-potassium-containing fluid, fresh blood products within a week and sodium bicarbonate infusion, which resulted in an adequate acid-base and electrolyte balance during the operation. Of interest in that case is the fact that the authors used non-potassium-containing 0.9% saline during SLKT. Recent studies on balanced crystalloids have revealed the benefits of low incidence of hyperchloremic metabolic acidosis and renal vasoconstriction, implicating less kidney injury [
6]. In addition, a more recent randomized controlled study reported that the risk of hyperkalemia in KT recipients receiving acetate-buffered balanced solution was not significantly higher than in KT recipients receiving 0.9% saline, although the former solution contains potassium [
8]. Therefore, in light of this information, we changed our primary fluid during LT and KT from 0.9% saline to acetate-buffered balanced crystalloid (i.e., Plasma solution A).
In 2002, Huh et al. [
7] reported two cases of SLKT. One involved a deceased donor and the other involved a living donor. Authors used ioRRT in the living donor case, and a clot formation in the filter disabled the system two hours after initiation. They replaced the filter, and the system stopped again after another two hours. The last period of anesthesia was maintained well without ioRRT. Theoretically, ioRRT actually has several complications. Above all, ioRRT has a risk of filter clotting. Unfortunately, systemic anticoagulation has a risk of increased bleeding tendency. It is difficult to stay at an appropriate point between pro-coagulation and anti-coagulation in patients with end-stage liver disease because they already have a drifting balance of coagulation. Recently, a regional anticoagulation technique with circuit inborn citrate was invented as an alternative. Other reported complications of ioRRT include hypothermia, vascular access dysfunction, fluid and electrolyte balance errors and, rarely, mechanical problems. Financial issues should also not be ignored, because ioRRT has been reported to consume a significant portion of national medical insurance resources in Korea.
As a next step, we searched international reference databases and found a few studies on the use of ioRRT in LT and SLKT. In the early 2000s, there were sporadic case reports and experience reports prepared by a single center or authors with a small-sized study population, addressing the possibility of better intraoperative management of metabolic and acid-base homeostasis with ioRRT. In 2011, Parmar et al. [
9] reported a retrospective cohort study of ioRRT in 72 LT recipients, and noted that there was no difference in post-operative complication rates between ioRRT and non-ioRRT groups, despite the fact that the ioRRT group had a higher pre-operative disease severity and CRRT ratio. However, this report was limited by the failure to incorporate matching between the two groups. In 2014, Agopian et al. [
10] reported a retrospective cohort study of ioRRT in 407 LT and 93 SLKT recipients who were already receiving preoperative CRRT. Among them, 401 patients did not receive ioRRT, 70 patients received planned ioRRT, and 29 patients received emergent ioRRT. Perioperative variables were analyzed between these three groups, and the authors observed similar intraoperative complication rates between the planned-ioRRT group and non-ioRRT group, despite the fact that preoperative disease severity variables were significantly worse in the planned-ioRRT group. From this, they suggested that these intraoperative outcomes, observed to be better than expected in the planned-ioRRT group, were in fact due to the utilization of ioRRT. In addition, they observed significantly lower intraoperative complication rates in the planned-ioRRT group than in the emergent-ioRRT group, despite the fact that preoperative disease severity variables were similar in the two groups. From this, the authors also suggested that the inferior intraoperative results in the emergent-ioRRT group could be due to not initiating ioRRT as a planned intervention.
As was demonstrated, most studies on the use of ioRRT are mainly observational or retrospective, and the necessary strong evidence does not exist so far. Fortunately, according to clinicaltrials. gov, the first large multicenter, prospective randomized controlled trial is in process by Bagshaw et al., which began in 2012. This study is designed to compare ioRRT during LT with standard supportive therapy in patients with a glomerular filtration rate (GFR) of less than 60 ml/min. The results are preliminary and the study population is limited to non-pre-transplant CRRT patients receiving LT alone; nevertheless, the study may become a cornerstone for ioRRT research and may affect the future management of high-MELD score recipients in the future.
Simultaneous liver and kidney transplantation
As a last step in this study, we searched literature databases for SLKT listing criteria. We realized that almost all of the SLKT recipients in Korea, including the patients from our cases, showed lower MELD scores as compared with recipients in the US. In living donor SLKT, the availability of simultaneous donors exerts more leverage on the decision of SLKT than the nature of the disease itself. However, even in the deceased donor case (case B), kidney function was enough to filtrate body wastes without RRT (SCr 1.58 mg/dl). This may be attributed to the current non-existence of SLKT listing criteria in Korea. In the US, there are a few consensus guidelines on SLKT listing criteria, which have been largely made and revised using Organ Procurement and Transplantation Network (OPTN) data. However, they are, by all means, not perfect and there are still many obstacles to be solved.
First, currently there is no consensus on the definitions of AKI and chronic kidney disease (CKD) in the setting of LT. It is well known that SCr, the classic biomarker, can lead to overestimation of the true kidney function in end-stage liver disease recipients. However, the feasibility and widespread laboratory availability of SCr has made it to form the basis for estimating the degree of renal dysfunction, even in LT and SLKT. For now, the most notable definition of renal dysfunction is the Risk, Injury, Failure, Loss, End-stage kidney disease (RIFLE) 5 stage criteria developed in 2004, which was modified to a three-stage system by the Acute Kidney Injury Network (AKIN) in 2007. Kidney Disease: Improving Global Outcomes (KDIGO) in 2012 added a last touch to the RIFLE criteria by formulating albuminuria criteria and applying a time frame to the SCr criteria. Furthermore, to define renal dysfunction in LT recipients, a working party by the Acute Dialysis Quality Initiative (ADQI) and International Ascites Club in 2011 suggested a proposal to apply the RIFLE criteria to define AKI and CKD in patients with cirrhosis, irrespective of the cause [
11]. New candidates for biomarkers, such as cystatin C and neutrophil gelatinase-associated lipocalin (NGAL), are currently under investigation. A new definition of renal dysfunction made up by those novel biomarkers is needed to create an appropriate SLKT listing criteria.
Second, key factors that determine non-recovery of kidney function after LT remain poorly defined. Recently, Sharma et al. [
12] reported a retrospective review analyzing 2,112 adult deceased donor LT recipients who received acute RRT for less than 90 days before LT. The authors concluded that native renal function was recovered in the majority of patients within six months post-transplant, with the cumulative risk of renal non-recovery being 8.9%. According to the study by Sharma et al., risk factors of renal non-recovery were age at LT, longer duration of RRT, retransplant and pre-LT diabetes. Although it is a good option for a patient who has both end-stage liver and kidney disease, SLKT should not be performed in patients in whom renal dysfunction is expected to disappear after LT. According to literatures, in the past decade in the US, more than 30,000 adults were added to the KT waiting list annually, but only 55–60% eventually received a deceased kidney graft. During that same time period, 13–15% of the individuals on the waiting list died while waiting for a transplant and 3–10% of them were removed from the list due to high disease severity. It should be noted that organ shortage is apparent in Korea, too. Further research on eligible risk factors is needed to avoid overzealous listing for SLKT.
Third, the appropriate time to initiate RRT in recipients waiting for SLKT is unknown. In the first national survey of practice patterns of SLKT in the US by OPTN, 70% of centers used dialysis duration as a criterion to determine the need for SLKT, whereas 30% of centers used AKI duration [
1]. However, there is still no guideline regarding when to start RRT in recipients waiting on a list. The decision of when to begin RRT is especially difficult to make in cases in which recipients develop AKI. Thus, a consensus on this decision is essential to build an acting SLKT criteria that embodies all recipients with or without RRT.
Lastly, there are debates regarding the true benefits of SLKT. In a review based on OPTN data by Formica et al. [
13], recipients undergoing LT with SCr > 2.5 mg/dl or pre-transplant dialysis time of > 2 months have 81.1% one-year survival rate and 65.9% five-year survival rate. SLKT increased this outcome to only 86.2% and 70.1%, respectively. More recent study data based on a propensity score-analysis of OPTN data concluded that survival benefit may even be as little as one month at five years after transplantation [
14]. In addition, transplantation of a kidney graft in recipients of LT with a high risk of mortality can be a waste of scarce organs. In a single-center study consisting of 169 patients with a MELD score > 40, futile LT outcomes (defined as three-month mortality or in-hospital mortality) occurred in over 22% of those involved in the study. In another analysis using the national database, patients with MELD score > 40 were more than twice as likely to die within 30 days of transplant than those with MELD scores of less than 30. According to them, futility predictive factors included age > 60 years, body mass index > 30 kg/m
2, a pre-transplant requirement for ICU care or life support and the presence of multiple comorbidities [
15]. The overall one-year kidney graft survival after SLKT was 77.2%, while graft survival after KT alone was 89.3%, following OPTN data in a similar study. Thus, the additory benefit from SLKT over LT alone should be better estimated and balanced with the disadvantage of patients waiting for KT alone.
Despite all of the limitations above, several consensus guidelines for SLKT listing criteria were made and published by several researchers as well as associations and government divisions in the US. The most notable ones are those from two big consensus conferences by OPTN. The first one was published by Eason et al. in 2008 and the second one by Nadim et al. in 2012 [
1]. The latter one adopted and modified the definition of AKI and CKD by KDIGO, and consisted of persistent AKI for more than four weeks, CKD for three months and metabolic diseases that affect both the liver and kidney. Recently, OPTN pronounced a new SLKT allocation criteria in 2016 [
13]. Those criteria are as follows. Metabolic diseases requiring SLKT (for example, primary hyperoxaluria), sustained AKI (defined as eGFR < 25 ml/min for six consecutive weeks) and/or CKD (defined as eGFR < 60 ml/min for> 90 days prior to listing and < 30 ml/min at the time of listing). All eGFR values are based on a six-variable Modified Diet in Renal Disease (MDRD6) formula. The guideline in 2016 also incorporated the concept of a “safety net,” which means prioritizing the patients who received LT to the kidney graft listing, in case of renal non-recovery. It was suggested that the addition of this component may affect the decision to undergo SLKT by removing the concern that if a patient does not receive the SLKT, that they will remain on dialysis and suffer a worse outcome.
In summary, we searched reported references to figure out the necessity of ioRRT in LT and SLKT. We also searched references to make an appropriate decision on the performance of SLKT, instead of LT alone. As we discussed above, great challenges are expected following the adoption of the MELD scoring system. It is not just a simple matter of increasing renal dysfunction; there are bundles of problems to be solved, like a domino effect. Utilization of novel biomarkers in LT recipients, the development of a more precise formula to detect kidney injury earlier; locating more risk factors on post-LT renal non-recovery; analyzing the effects of ioRRT on the renal recovery and determining the benefits of ioRRT in either living or deceased grafts; investigating complication reports of ioRRT; building a national statistics of SLKT and ioRRT; estimating the additory benefit from SLKT and the disadvantage of patients on KT waiting list; and finally, establishing our own consensus criteria for both ioRRT and SLKT are all important considerations in the post-MELD era of LT anesthetic portion of care. Further research is needed indeed in next decade.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download