Abstract
Background
Droperidol (DHB) reportedly reduces the dose of propofol needed to achieve hypnosis when anesthesia is induced and decreases the bispectral index (BIS) in propofol-sedated patients during spinal anesthesia. We reported previously that supplemental DHB decreased the BIS after the administration of sevoflurane and remifentanil. This study investigated the effect of DHB on desflurane (DES) consumption in a clinical setting.
Methods
We conducted a prospective, randomized double-blinded study of 35 women with American Society of Anesthesiologist physical status I or II who underwent a mastectomy. Either DHB (20 µg/kg) or a saline placebo was administered to patients 30 min after the induction of anesthesia. A blinded anesthesiologist maintained a BIS value of 50 during anesthesia by modulating inhaled DES concentrations that changed 0.5% at 2.5 min intervals and maintained analgesia via the constant administration of remifentanil by referring to vital signs. The primary endpoint was the effect of DHB on DES consumption. The secondary endpoints included blood circulatory parameters, the time from the end of surgery to extubation, and discharge time between the groups.
Droperidol (DHB) is a dopamine D2 receptor antagonist and a commonly used tranquilizer and sedative. Many physicians, including anesthesiologists, administer DHB to prevent postoperative nausea and vomiting (PONV) and to treat delirium or aggression in the intensive care unit.
DHB can prolong the QTc interval [1]; thus, the US Food and Drug Administration black box warning [2] discourages the use of DHB by anesthesiologists, even in Japan. However, some reports indicate that low-dose DHB does not increase the risk of polymorphic ventricular tachycardia or death in surgical patients [3]. Furthermore, one study suggested that even 5HT3 antagonists, which are commonly used as anti-emetics, prolong the QTc interval [4].
DHB reduces the dose of propofol needed to achieve hypnosis when inducing anesthesia [567], and it decreases the bispectral index (BIS) in propofol-sedated patients during spinal anesthesia [8]. We reported previously that administering supplemental DHB reduced the BIS during general anesthesia with sevoflurane and remifentanil [9] and hypothesized that DHB could reduce the desflurane (DES) concentration needed for an adequate anesthesia depth using the BIS.
In the current study, we investigated the sparing effect of DHB on the consumption of DES in a clinical setting.
This study was a prospective, randomized double-blinded trial conducted from March to September 2014 at Medical Hospital of Tokyo Medical and Dental University (Bunkyo-ku, Tokyo, Japan). Ethical approval for this study (Ethical Committee No. 1741) was provided by the Ethical Committee of Tokyo Medical and Dental University, Tokyo, Japan. After obtaining approval from the Institutional Review Board, we recruited 42 women (age, 38–77 years) with an American Society of Anesthesiologist physical status of I or II who underwent a mastectomy or quadrantectomy. Each participant gave written informed consent. The exclusion criteria included abuse of alcohol or drugs, psychiatric disease, and hypersensitivity to DHB or DES. Patients were allocated randomly to the DHB or saline group. The study drugs were drawn into identical 2 ml syringes, labelled "anti-emetic" (DHB was diluted to 2 ml in normal saline) by a nurse who was not part of the study, and handed to the anesthesiologist. The patient and investigators were blinded to the study medication.
General anesthesia was induced with 1.5 mg/kg of propofol, 0.6 mg/kg of rocuronium, and 0.2 µg/kg/min of remifentanil. All patients were intubated and placed on mandatory ventilation. The patients were infused continuously with 7 µg/kg/min of rocuronium and 0.2 µg/kg/min of remifentanil during surgery. The total gas flow was 2 L/min with 40% oxygen as the carrier gas. A blinded anesthesiologist maintained a BIS value of 50 during anesthesia by modulating the inhaled DES concentration, which changed 0.5% at 2.5 min intervals, and by referring to the vital signs.
Either DHB (20 µg/kg) or a saline placebo was administered to the patients 30 min after the induction of anesthesia. The BIS value was maintained at 50 by a blinded anesthesiologist after administering either DHB or saline. All anesthetics were discontinued at the end of surgery. The endotracheal tube was removed after confirming complete recovery of consciousness, adequate spontaneous ventilation, and a sufficient reflex against suctioning stimuli. The DES volume was calculated as follows:
The constant number at 20℃ was derived from the equation:
The total gas flow was 2 L/min with 40% continuous oxygen as the carrier gas. After surgery, the mean inhaled volume of DES was calculated every 15 min to maintain a BIS value of 50 by modulating the DES concentrations inhaled during 0.5% changes at 2.5 min intervals by referring to an anesthesia chart. The total DES used during anesthesia was summarized for all patients. The cost of the inhaled DES (1 ml DES = 44.9 Japanese yen) was calculated. After the price of DHB was added (1 vial = 112 Japanese yen), we compared the cost of DES between the two groups.
The primary endpoint was the saving effect of DHB on DES consumption. The secondary endpoints included heart rate, blood pressure, time from the end of surgery to extubation, and discharge time between the groups. The frequency of and required treatment for PONV were evaluated from the medical records on postoperative day 1.
A pilot trial to determine the effect of DHB on the BIS value showed significant differences [9]. After the dose responsiveness analysis, we hypothesized that administering DHB reduced the DES concentration required by at least 25% from baseline. Thus, the assumed maximal difference in mean concentration was approximately 1% (4% in the control group and 3% in the DHB group), and the standard deviation was 0.5% in each group. A minimum of 36 patients was estimated to be needed to demonstrate a clinically significant difference after supplemental DHB administration with α = 0.05 and power = 0.8. We recruited 45 patients with a 20% expected dropout rate. The calculation was performed using PASS 2008 (Number Cruncher Statistical Systems, Kaysville, UT, USA).
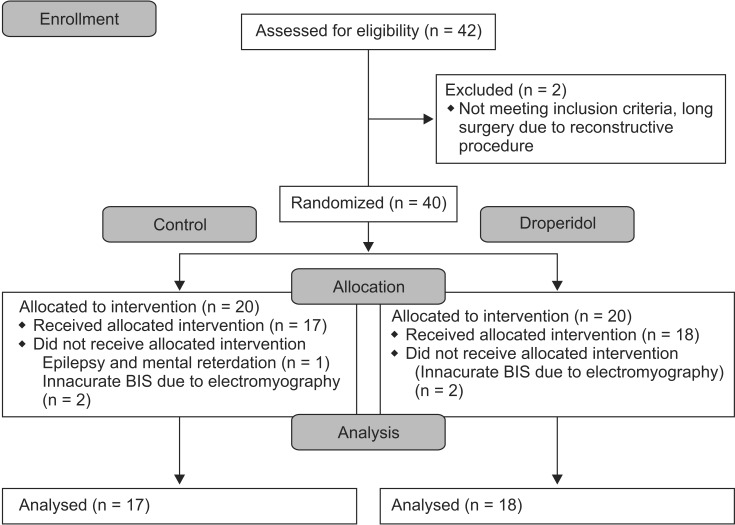
Seven patients were excluded from the investigation (two for long surgery due to reconstructive procedures, one for epilepsy and mental retardation, and four for inaccurate BIS values due to electromyography without rocuronium throughout surgery) (Fig. 1). Thus, data from 35 patients were analyzed. The patients' demographic characteristics, duration of surgery and anesthesia, and blood circulatory parameters before DHB administration did not differ between the groups (Table 1).
Heart rate and blood pressure remained unchanged in each group before and after DHB administration, and did not differ between the two groups. The group that received supplemental DHB required a significantly lower concentration of inhaled DES to maintain a BIS of 50 than did the placebo group (Fig. 2). The mean volumes of DES consumed were 27.2 ± 6.0 ml in the DHB group and 41.3 ± 9.5 ml in the control group (P < 0.05; Fig. 3). The mean cost of DES was $10.90 ($7.20–16.50 + one vial of DHB = 123 Japanese yen for each patient, $1 = 124.6 Japanese yen) in the DHB group and $14.90 ($8.80–21.20, $1 = 124.6 Japanese yen) in the control group.
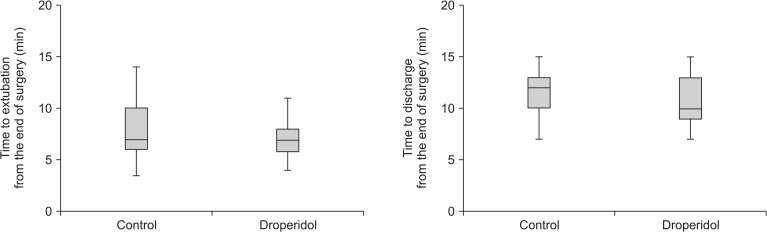
The durations from the end of surgery to extubation and discharge did not differ between the groups (Fig. 4). Fewer patients suffered from PONV in the DHB group (four cases of nausea and one of vomiting) than in the placebo group (eleven cases of nausea and three of vomiting), but the difference was not significant.
Intraoperative administration of DHB reduced the DES concentration required to maintain a BIS value of 50 without any apparent changes in cardiovascular parameters. Recovery from general anesthesia was not affected by DHB. Anesthesiologists perpetually explore the appropriate depth of anesthesia for each patient during surgery, which is evaluated by the BIS value, heart rate, and blood pressure. Deep general anesthesia measured using the BIS value has been associated with increased mortality in observational studies [1011121314]. Maintaining an adequate depth of anesthesia is important for anesthesiologists.
DES was commercialized only 4 years ago in Japan, which is 21 years after sevoflurane became commercially available. The current prices of both agents per unit volume are similar; however, the minimum alveolar concentration (MAC) of DES is approximately three times higher than that of sevoflurane. Thus, the actual cost of using DES as an anesthetic is about three times higher than that of using sevoflurane. The purchase price of a 25 mg vial of DHB is about $0.98. If supplemental use of DHB augments the anesthetic potency of DES without adverse effects, including recovery time, the consumption and cost of DES can be reduced safely.
Appropriate drug combinations are beneficial in anesthesia. General anesthetics commonly induce circulatory depression, and inducing anesthesia with a high concentration can cause severe adverse effects [15]. Higuchi et al. [16] reported that premedication with clonidine decreased the required dose of propofol to induce anesthesia. Wajima et al. [17] demonstrated that oral premedication with tizanidine reduced the MAC of sevoflurane by 18% in human adults, whereas gabapentin failed to modify the MAC of isoflurane in cats [18]. DHB reduced the dose of propofol required to induce anesthesia [5], and sevoflurane decreased the BIS value during general anesthesia [9].
In this study, we administered small doses of DHB as prophylaxis for PONV [19]. Female sex is a risk factor for PONV [20]. Relatively few patients in the current study failed to demonstrate a significant anti-emetic effect of supplemental DHB, although DHB was advantageous for maintaining the depth of general anesthesia. We occasionally experienced a patient with a sudden decrease in the BIS value immediately after administering DHB. Although dexamethasone is a well-known and appropriate drug to prevent PONV [21], it can impair glucose tolerance [22].
The risk of intraoperative awareness should be addressed. The BIS value is a popular monitoring tool to measure the depth of anesthesia, and BIS-guided anesthesia has significantly reduced the incidence of intraoperative recall in surgical patients at high risk for awareness [23]. Russell [24] reported that the BIS value is poorly correlated with intraoperative responsiveness. However, no solution has been reported to prevent intraoperative awareness, but none of the patients reported intraoperative awareness during this study.
Our study had several potential limitations. First, all participants were women, and the results could vary by sex, considering that PONV is more frequent in female patients. Second, the patients' age range was rather wide, which could increase the standard deviations in the data. Third, we used a single DHB dose by body weight and therefore did not evaluate the effects by dose, for which further investigation is required. Finally, this was a single-institution study, which may have affected the results.
In summary, a 20 µg/kg dose of DHB significantly reduced the DES concentration needed to maintain a constant BIS value. Our results suggest that DHB supplementation can be used to safely curtail the cost of general anesthesia.
Acknowledgments
We thank Dr. Mamoru Yamamoto (Department of Anesthesiology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan) for his help with this study.
References
1. Nakao S, Hatano K, Sumi C, Masuzawa M, Sakamoto S, Ikeda S, et al. Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients. Anesth Analg. 2010; 110:775–779. PMID: 20185656.

2. Halloran K, Barash PG. Inside the black box: current policies and concerns with the United States Food and Drug Administration's highest drug safety warning system. Curr Opin Anaesthesiol. 2010; 23:423–427. PMID: 20446348.

3. Nuttall GA, Malone AM, Michels CA, Trudell LC, Renk TD, Marienau ME, et al. Does low-dose droperidol increase the risk of polymorphic ventricular tachycardia or death in the surgical patient? Anesthesiology. 2013; 118:382–386. PMID: 23291623.

4. Staikou C, Stamelos M, Stavroulakis E. Impact of anaesthetic drugs and adjuvants on ECG markers of torsadogenicity. Br J Anaesth. 2014; 112:217–230. PMID: 24305646.

5. Adachi YU, Uchihashi Y, Watanabe K, Satoh T. Small dose midazolam or droperidol reduces the hypnotic dose of propofol at the induction of anaesthesia. Eur J Anaesthesiol. 2000; 17:126–131. PMID: 10758457.

6. Schaub I, Lysakowski C, Elia N, Tramèr MR. Low-dose droperidol (≤1 mg or ≤15 μg kg-1) for the prevention of postoperative nausea and vomiting in adults: quantitative systematic review of randomised controlled trials. Eur J Anaesthesiol. 2012; 29:286–294. PMID: 22488335.
7. Griffiths JD, Gyte GM, Paranjothy S, Brown HC, Broughton HK, Thomas J. Interventions for preventing nausea and vomiting in women undergoing regional anaesthesia for caesarean section. Cochrane Database Syst Rev. 2012; (9):CD007579. PMID: 22972112.

8. Nakayama M, Kanaya N, Ichinose H, Yamamoto S, Namiki A. Intravenous droperidol causes a reduction in the bispectral index in propofol-sedated patients during spinal anesthesia. Anesth Analg. 2003; 96:765–768. PMID: 12598260.

9. Adachi YU, Tanaka K, Suzuki S, Nishiwaki K, Matsuda N. Intravenous droperidol decreases the bispectral index during general anesthesia with sevoflurane and remifentanil. Masui. 2013; 62:71–74. PMID: 23431897.
10. Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005; 100:4–10. PMID: 15616043.

11. Lindholm ML, Träff S, Granath F, Greenwald SD, Ekbom A, Lennmarken C, et al. Mortality within 2 years after surgery in relation to low intraoperative bispectral index values and preexisting malignant disease. Anesth Analg. 2009; 108:508–512. PMID: 19151279.

12. Leslie K, Myles PS, Forbes A, Chan MT. The effect of bispectral index monitoring on long-term survival in the B-aware trial. Anesth Analg. 2010; 110:816–822. PMID: 19910621.

13. Kertai MD, Pal N, Palanca BJ, Lin N, Searleman SA, Zhang L, et al. Association of perioperative risk factors and cumulative duration of low bispectral index with intermediate-term mortality after cardiac surgery in the B-Unaware Trial. Anesthesiology. 2010; 112:1116–1127. PMID: 20418692.

14. Kertai MD, Palanca BJ, Pal N, Burnside BA, Zhang L, Sadiq F, et al. Bispectral index monitoring, duration of bispectral index below 45, patient risk factors, and intermediate-term mortality after noncardiac surgery in the B-Unaware Trial. Anesthesiology. 2011; 114:545–556. PMID: 21293252.

15. Torri G. Inhalation anesthetics: a review. Minerva Anestesiol. 2010; 76:215–228. PMID: 20203550.
16. Higuchi H, Adachi Y, Dahan A, Olofsen E, Arimura S, Mori T, et al. The interaction between propofol and clonidine for loss of consciousness. Anesth Analg. 2002; 94:886–891. PMID: 11916791.

17. Wajima Z, Yoshikawa T, Ogura A, Imanaga K, Shiga T, Inoue T, et al. Oral tizanidine, an alpha2-adrenoceptor agonist, reduces the minimum alveolar concentration of sevoflurane in human adults. Anesth Analg. 2002; 95:393–396. PMID: 12145058.
18. Reid P, Pypendop BH, Ilkiw JE. The effects of intravenous gabapentin administration on the minimum alveolar concentration of isoflurane in cats. Anesth Analg. 2010; 111:633–637. PMID: 20547821.

19. Purhonen S, Kauko M, Koski EM, Nuutinen L. Comparison of tropisetron, droperidol, and saline in the prevention of postoperative nausea and vomiting after gynecologic surgery. Anesth Analg. 1997; 84:662–667. PMID: 9052320.

20. Eberhart LH, Högel J, Seeling W, Staack AM, Geldner G, Georgieff M. Evaluation of three risk scores to predict postoperative nausea and vomiting. Acta Anaesthesiol Scand. 2000; 44:480–488. PMID: 10757586.

21. Voigt M, Fröhlich CW, Waschke KF, Lenz C, Göbel U, Kerger H. Prophylaxis of postoperative nausea and vomiting in elective breast surgery. J Clin Anesth. 2011; 23:461–468. PMID: 21911192.

22. Nazar CE, Lacassie HJ, López RA, Muñoz HR. Dexamethasone for postoperative nausea and vomiting prophylaxis: effect on glycaemia in obese patients with impaired glucose tolerance. Eur J Anaesthesiol. 2009; 26:318–321. PMID: 19401661.

23. Punjasawadwong Y, Boonjeungmonkol N, Phongchiewboon A. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. 2007; (4):CD003843. PMID: 17943802.

24. Russell IF. The ability of bispectral index to detect intra-operative wakefulness during total intravenous anaesthesia compared with the isolated forearm technique. Anaesthesia. 2013; 68:502–511. PMID: 23521699.

Fig. 2
Changes in the dial setting of the desflurane vaporizer. The data are given as means ± SD. *P < 0.05 vs. the control group.
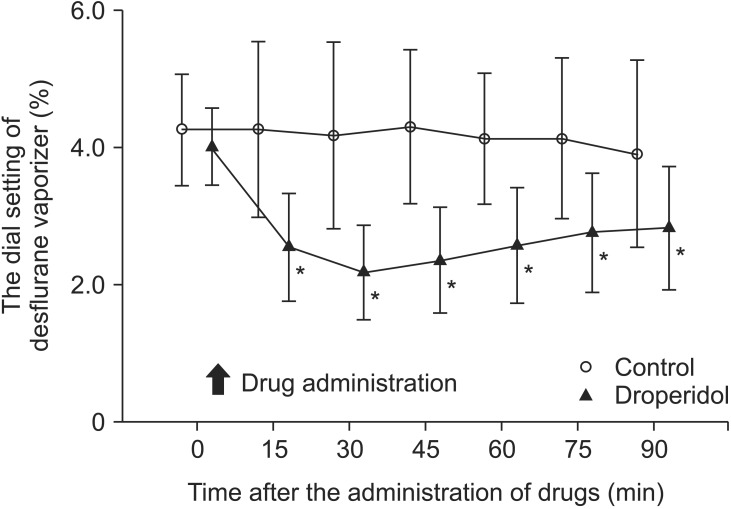
Fig. 3
Calculated volume of desflurane consumed during the observation period. The groups differed significantly. Data were expressed as median, minimum, first interquartile, third interquartile and maxium values. *P < 0.05.
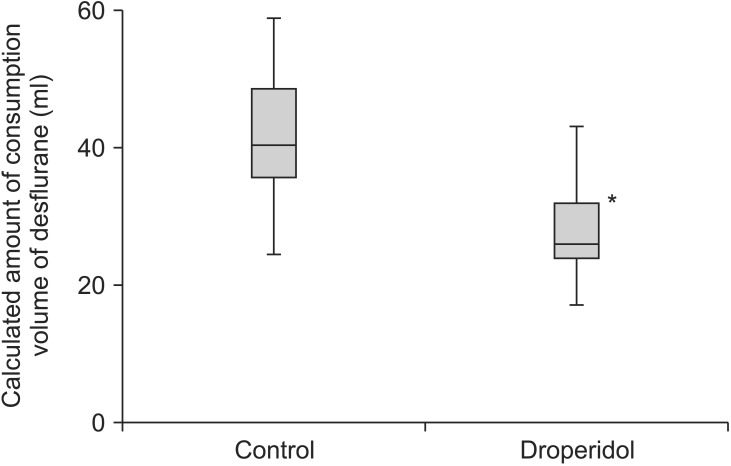
Fig. 4
Box plot showing the time to extubation and discharge after surgery. Data were expressed as median, minimum, first interquartile, third interquartile and maxium values. The groups did not differ significantly.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download