Abstract
Background
The current study evaluated the hemodynamic effects of different types of pneumatic compressions of the lower extremities during anesthesia induction. In addition, the hemodynamic effects were compared between patients older than 65 age years and those aged 65 years or younger.
Methods
One hundred and eighty patients (90: > 65 years and 90: ≤ 65 years) were enrolled. Each age group of patients was randomly assigned to one of three groups; Group 1 (no compression), Group 2 (sequential pneumatic compression), and Group 3 (sustained pneumatic compression without decompression). Invasive blood pressure, cardiac index (CI), and stroke volume variation (SVV) were measured.
Results
In patients aged ≤ 65 years, mean arterial pressure (MAP) and CI were significantly higher and SVV was lower in Group 3 compared to Group 1 before tracheal intubation, but there were no differences between Groups 1 and 2. However, there were no differences in MAP, CI, and SVV among the three groups in patients aged > 65 years. The number of patients who showed a MAP < 60 mmHg was less in Group 3 than Group 1 in patients aged ≤ 65 years, but not in patients aged > 65 years.
Conclusions
Sustained pneumatic compression of the lower extremities has more hemodynamic stabilizing effects compared to sequential compression during anesthesia induction in patients aged 65 years or younger. However, no difference between methods of compression was observed in patients older than 65 years.
Modern anesthetics have vasodilatory effect with minimal myocardial depression. The effect of anesthetics decrease systemic vascular resistance (SVR) and venous return. Furthermore, anesthesia decrease sympathetic activity before surgical stimulation [1,2]. Because anesthetics redistribute the blood from the central to the peripheral compartment secondary to vasodilatation for the reasons above, hemodynamic instability is common during the induction of general anesthesia and preparation for operation. The redistribution may decrease cardiac preload and results in a decrease in the stroke volume (SV) according to the Frank-Starling’s law. Also, positive pressure ventilation decreases cardiac output result from effects on the right heart [3]. Consequently, blood pressure drops result from decreased cardiac output and SVR during general anesthesia induction.
In clinical practice, large volumes of fluid or short-acting cardiovascular active agent such as ephedrine may be administered to counteract anesthesia-induced hypotension. However, many previous reports have shown the risks of fluid overload although the role of perioperative fluid management remains under debate [4–7]. Additionally, the administration of ephedrine has a risk of myocardial ischemia due to severe tachycardia [8]. Therefore, prevention of the redistribution of blood during anesthesia might be a good option to avoid hemodynamic instability and the adverse effects of intravenous fluids and cardiovascular active agents. Although Trendelenburg positioning or passive legraising is a simple maneuver to increase the blood in the central compartment to restore cardiac preload, it is not feasible in many clinical situations. However, external compression of the lower extremities decreases the peripheral pooling of blood and augments venous return to increase central blood volume. Many previous reports have shown that the effects of pneumatic compression on the prevention of anesthesia- or position-related hypotension resulted from the peripheral redistribution of central blood volume [9–12]. Several types of pneumatic compression of the lower extremities, including pneumatic splint, intermittent compression, peristaltic compression, sequential compression, and military anti-shock trouser (MAST) are established interventions for various indications such as lymphedema, postthrombotic ulcers, arterial claudication, deep vein thrombosis, and severe trauma [13,14].
We hypothesized that the use of pneumatic compression of the lower extremities would decrease hemodynamic instability during anesthesia induction and the effects may be different by the types of compression. In addition, the effects may be predominant in elderly patients. The primary purpose of this study was to compare the effects of pneumatic compression on the incidence of hypotension and hemodynamic variables by the types of compression during anesthesia induction. The secondary outcome of the study was comparison of the hemodynamic effects of pneumatic compression between patients older than 65 years and aged 65 years or younger.
This prospective randomized controlled study was approved by the Institutional Review Board of author’s institution, and written informed consent was obtained for all participants. One hundred and eighty patients, American Society of Anesthesiologists physical status I or II and aged 20 to 85 years, undergoing elective major operations that required invasive monitoring were enrolled. The patients that presented hypertension, cardiac arrhythmia, heart failure, vascular diseases, renal failure, CNS disorders, diabetes mellitus, alcohol or drug abuse, or a body mass index of < 18 or > 35 were excluded. Subjects were separated into those older than 65 years and those aged 65 years or younger initially. Each age group patients was randomly assigned by a computer-generated random number to one of three groups at preanesthetic visit according to the types of pneumatic compression of the lower extremities: Group 1 (control, no pneumatic compression), Group 2 (sequential pneumatic compression), and Group 3 (sustained pneumatic compression without decompression).
The anesthetic regimen was standardized with total intravenous anesthesia for all patients. Patients fasted for 8-12 h before surgery and received maintenance fluids. Glycopyrrolate 0.2 mg was administered intravenously before transport to the operating theater. Before induction of anesthesia, a 20-G arterial catheter (Arterial cannula, Becton Dickinson Infusion Therapy System, USA) was inserted into the radial artery in the non-dominant hand after infiltration anesthesia with 2% lidocaine. The radial artery catheter was connected to the FloTracTM transducer (Edwards Lifesciences, USA) coupled to both an anesthesia workstation (Primus InfinityⓇ Empowered, Dräger, Germany) and EV1000TM (software version 1.5, Edwards Lifesciences) for hemodynamic measurements such as invasive blood pressure (IBP), SV, cardiac index (CI), and stroke volume variation (SVV). Pressure transducers were zeroed at the mid-axillary level to atmospheric pressure. Additionally, patients were monitored for electrocardiogram, pulse oximetry, temperature, capnography, peripheral nerve stimulator, and bispectral index (BIS). Baseline hemodynamic variables were measured before anesthesia induction. If the SVV of a patient was more than 12%, the patient received 100 ml of saline and 50 ml increments until SVV presented less than 12%. Patients were administered 2% lidocaine 2 ml intravenously with a tourniquet to prevent injection pain of propofol.
For the compression of the lower extremities, a pneumatic compression device (MK-400L, DS Maref, Korea) was used. The sleeves of the compression device, which has six air chambers, were applied from the foot to the thigh in all patients and compressed with 40 mmHg of preset pressure circumferentially. After measurement of the baseline hemodynamic variables, pneumatic compression was started. The pneumatic compression was sequentially applied from the foot to the thigh to produce a milking action in Group 2. The total compression time of the device was 67 s with 10 s of compression time in each chamber and 7 s of full chamber compression time. One cycle of sequential compression was 80 s with 13 s of decompression time. In Group 3, 40 mmHg of sustained compression of the lower extremities was maintained for the study periods. The compression pressure was not applied in Group 1 as the control group.
After BIS, vital signs, and hemodynamic variables were measured at the baseline, and anesthesia was induced by propofol (Fresofol MCTⓇ 2%, Fresenius Kabi, Austria) and remifentanil (UltivaⓇ, GlaxoSmithKline, Italy). Propofol and remifentanil were infused using an effect-site target-controlled infusion pump (OrchestraⓇ Base Primea, Fresenius Vial, France) with the Marsh and Minto models, respectively. Initially, patients were induced with propofol 4 μg/ml and remifentanil 3 ng/ml of the effect-site concentration. After patients lost consciousness, 1.0 mg/kg of rocuronium was administered. The patients were manually ventilated via a face mask with air in oxygen and the investigators have tried to keep a fixed tidal volume (TV) of 8–10 ml/kg under spirometric monitoring. Orotracheal intubation was performed 3 min after loss of consciousness (LOC). After endotracheal intubation, controlled mechanical ventilation was maintained with a TV of 8 ml/kg and an inspiratory to expiratory ratio of 1 : 2 without positive end-expiratory pressure. The ventilation frequency was set to maintain an end-tidal PCO2 range of 34–38 mmHg. Two minutes after endotracheal intubation, the effect-site concentrations of propofol and remifentanil were decreased to 2.5 μg/ml and 2.0 ng/ml, respectively.
To evaluate the effects of the types of pneumatic compression on hemodynamic variables, IBP, heart rate (HR), CI, and SVV were measured at pre-anesthesia (baseline), at LOC, and at 1, 2, and 3 min after LOC and measured for 5 min with a 1-min interval after intubation. Additionally, BIS was recorded at the same time periods. If a patient showed a mean arterial pressure (MAP) of < 60 mmHg or BIS > 60, the patient received 10 mg of ephedrine or the propofol concentration was increased in 0.5 μg/ml increments, respectively. The number of patients who showed an MAP of < 60 or > 130 mmHg and CI of < 2.2 or >5.0 L/min/m2 was recorded.
We considered a clinically significant difference in MAP, which was regarded as the primary endpoint, to be ≥ 10 mmHg. It was ascertained that 26 patients were required in each group to show a difference in MAP of 10 mmHg for an expected standard deviation (SD) of 10 mmHg with a significance level of 0.05 (α = 0.05) and a power of 90% (β = 0.10). The sample size was calculated by one-way analysis of variance (ANOVA) using SigmaPlot 12.5 (Systat Software Inc., USA). To allow for attrition, the sample size was enlarged to 90 in each age group.
Statistical analysis was performed with SigmaPlot 12.5. Continuous variables such as patient demographics, BIS, and hemodynamic variables were analyzed with one-way ANOVA, and the incidence of hypotension was analyzed using the Chi-square test. The Holm-Sidak method was used for multiple comparisons after the ANOVA test. All data are expressed as mean ± SD. A P value of < 0.05 was considered statistically significant.
Six and three patients were excluded in patients aged > 65 years and ≤ 65 years, respectively. The data from 171 patients were analyzed (Fig. 1). All groups in each aged patients were comparable in terms of patient characteristics (Table 1). Before anesthesia induction, three patients aged ≤ 65 years and 4 patients aged > 65 years received crystalloid fluid to decrease SVV to less than 12%, and the mean infused volumes were 200 and 183 ml, respectively. There were no differences in MAP, HR, CI, SVV, and BIS at the preanesthetic baseline values among the three groups in both patients aged > 65 years and ≤ 65 years.
MAP and CI were significantly decreased after anesthesia induction compared with the baseline values until intubation in all patients groups. In patients aged ≤ 65 years, MAP and CI were significantly higher in the sustained pneumatic compression group (Group 3) compared with the control group (Group 1) from 1 min after loss of consciousness to just before endotracheal intubation, but there were no differences between Group 1 and Group 2 (sequential compression group) and Groups 2 and 3. In patients aged > 65 years, there were no differences in MAP and CI among the three groups (Figs. 2 and 3). SVV was increased after anesthesia induction in all patients. SVV was significantly lower in Group 3 than Groups 1 and 2 after anesthesia induction in patients aged ≤ 65 years. However, there were no differences among the three groups at any time points in patients aged > 65 years (Fig. 4). HR and BIS were not different by types of pneumatic compression at each time point in both ages of patients.
The number of patients who showed a MAP of < 60 mmHg was lower in Group 3 than Group 1 in patients aged ≤ 65 years, but there were no significant differences among the three groups in patients aged > 65 years. There were no differences in the number of patients with a CI < 2.2 L/min/m2 among the three groups in each age of patients. The number of patients who had MAP > 130 mmHg or CI > 5.0 L/min/m2 were similar among the three groups after endotracheal intubation (Table 2).
This study was conducted to compare the effects of two types of pneumatic compression of the lower extremities on hemodynamic variables during intravenous anesthesia induction. Furthermore, the effects were compared between patients older than 65 years and patients aged 65 years or younger. The authors hypothesized that sustained compression is more effective for preventing hemodynamic instability than sequential compression because venous blood can be pooled in the lower extremities during the decompression period of sequential compression. Because hemodynamic instability is more common and severe in elderly patients than younger patients during anesthesia induction, the authors speculated that the hemodynamic effects of pneumatic compression are greater in elderly patients. In the current study, sustained pneumatic compression more effectively prevented the decrease in MAP and CI and the increase in SVV than sequential pneumatic compression in patients aged ≤ 65 years. However, unlike the authors’ hypothesis, the hemodynamic effects of pneumatic compression were not observed in elderly patients regardless of types of compression.
Although there are several types of pneumatic compression, two types of pneumatic compressions, sequential and sustained compressions, were compared in this study. Sequential pneumatic compression is a variation of intermittent pneumatic compression, using sequential compression from the foot upwards and decompression cycles. Because intermittent pneumatic compression is most commonly used in the prevention of deep venous thrombosis, most previous studies that evaluated the effects of pneumatic compression on hemodynamic instability related to spinal anesthesia [9,15], operation position[11], and surgery [10,16] have used intermittent pneumatic compression. However, there has been no previous study that compared the effects by types of compression. It may be inferred from our results that sustained pneumatic compression may be more effective than intermittent pneumatic compression in perioperative hemodynamic instability related to spinal anesthesia, sitting position, and pneumoperitoneum. In clinical practice, sustained pneumatic compression of the lower extremities is used in severe traumatized patients or post-varicose vein operation. Sustained pneumatic compression is physically similar to the leg portion of MAST, but different in pressure. Because MAST is inflated with pressures of up to 100 mmHg, it can increase SVR as well as preload [17]. However, most previous studies that evaluated hemodynamic effects of pneumatic compression of the lower extremities were applied 40–60 mmHg [9,10,12,16]. SVR was not expected to increase similar as MAST with lower pressure (40 mmHg) application in our study. If a higher pressure was applied in both sustained and sequential compressions in the current study, more hemodynamic stabilizing effects may be expected through increases in preload and SVR. Although the use of a higher pressure as MAST is not optimal because it potentially impedes overall blood supply and venous return, highpressure compression may be applicable for short periods such as induction of anesthesia. One question remains whether lowpressure pneumatic compression can squeeze blood in the deep veins of the lower extremities. Therefore, further investigation is needed for an effective compression pressure.
Although intermittent pneumatic compression is a simple and reliable technique that increases the blood returning from the lower extremities, there have been few controlled studies to determine the increase in the central blood volume. Estimates of the recruited blood volumes from the lower extremities are variable in the literature. One study reported that an average of 107 ml of blood can be moved by 50 mmHg of sequential compression of the ankle and the calf [18]. Because thigh-high compression sleeves were used in the current study, a greater volume of venous return is expected than in a calf-high compression. In the other study, patients treated with thigh-high peristaltic pneumatic compression received 693 ml of crystalloids less than the placebo compression group [12]. These results suggest that pneumatic compression of the lower extremities significantly reduces the fluid demand result from increased central blood volume. Unger and Feiner [19] observed that intermittent pneumatic compression increased central venous pressures, pulmonary artery pressures, and pulse pressures. Although they did not measure stroke volume, they suggested that the increased pulse pressures reflected an increased stroke volume. In the current study, CI was decreased to 62% of the baseline value in control group patients but, to 73% in sustained compression group patients at just before intubation in patients aged 65 years or younger. The difference of CI between the two groups could be due to the difference in stroke volume because the heart rates were similar in the two groups. Furthermore, SVV, a parameter of predicting fluid responsiveness, was significantly less increased in patients with sustained compression compared with control patients.
In the current study, the hemodynamic stabilizing effects of sustained pneumatic compression were predominant in patients aged ≤ 65 years, but the effects were not observed in patients aged > 65 years. The authors speculated the reasons for the difference. Firstly, the volume of recruited blood from the lower extremities in elderly patients were less than those of younger patients because of decreased muscle mass of the lower extremities. Secondly, the anesthetics-induced cardiac depression effect was more severe in elderly patients. The effect masks the hemodynamic stabilizing effects of pneumatic compression because CI was more decreased in elderly patients than younger patients. Most previous studies that investigated the pneumatic compression of the lower extremities examined young patients with intermittent or sequential compression as Group 2 aged ≤ 65 years. The studies showed hemodynamic stabilizing effects unlike our results. The authors considered that the discrepancy may be caused by relatively lower pressure and higher age in the current study.
Fluid loading or administration of cardiovascular active agent such as ephedrine or phenylephrine to treat anesthesiainduced hypotension causes minimal risk in healthy patients. Because application of pneumatic compression is more complicating process in simple anesthetic induction, routine use of pneumatic compression is limited in healthy patients. However, administration of fluid or vasoactive drugs should be avoided in some cases. Although the effects of sustained pneumatic compression were only 10 mmHg of MAP differences observed and limited in aged < 65 years old, it may work as alternative technique for avoiding fluid or vasoactive drugs administration. In addition, hypertension should also be avoided as well as hypotension during anesthesia induction. In the current study, there were no differences the number of patients who had MAP > 130 mmHg among 3 groups. Also, the average MAP was similar after tracheal intubation although the incidence of hypotension was low in sustained pneumatic compression groups in patients aged ≤ 65 years. Therefore, application of sustained pneumatic compression would be a good option to prevent hypotension without increasing incidence of hypertension during anesthesia induction.
There are two limitations in the current study. First, the preanesthetic baseline values of SVV were used as a preload parameter during spontaneous breathing. SVV is considered to unreliable in patients with spontaneous breathing. However, most patients received mechanical ventilation with a fixed TV of 8–10 ml/kg under spirometric monitoring after propofol administration. Four percent of the patients (7 of 171) received fluid loading using preanesthetic SVV values before anesthesia induction in the current study. The main outcomes of the current study are hemodynamic changes after anesthesia induction and those before the beginning of operation. Therefore, the effect of preanesthetic SVV values and operation type were limited. Second, we could not determine why the results of the effects of sustained compression were different between elderly and younger patients. If we measured SVR, SV, and right ventricular volume using a pulmonary artery catheter and echocardiography, the cause may be clarified. In addition, the effects of compression pressure on SVR would be specified.
In conclusion, sustained pneumatic compression of the lower extremities has more hemodynamic stabilizing effects compared with sequential compression during intravenous anesthesia induction in patients aged 65 years or younger. However, the effects are not observed in patients older than 65 years.
References
1. de Wit F, van Vliet AL, de Wilde RB, Jansen JR, Vuyk J, Aarts LP, et al. The effect of propofol on haemodynamics: cardiac output, venous return, mean systemic filling pressure, and vascular resistances. Br J Anaesth. 2016; 116:784–9.
2. Sellgren J, Pontén J, Wallin BG. Characteristics of muscle nerve sympathetic activity during general anaesthesia in humans. Acta Anaesthesiol Scand. 1992; 36:336–45.

3. Lansdorp B, Hofhuizen C, van Lavieren M, van Swieten H, Lemson J, van Putten MJ, et al. Mechanical ventilation-induced intrathoracic pressure distribution and heart-lung interactions*. Crit Care Med. 2014; 42:1983–90.

4. Corcoran T, Rhodes JE, Clarke S, Myles PS, Ho KM. Perioperative fluid management strategies in major surgery: a stratified meta-analysis. Anesth Analg. 2012; 114:640–51.
5. Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002; 89:622–32.

6. Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg. 2006; 93:1069–76.

7. Boland MR, Noorani A, Varty K, Coffey JC, Agha R, Walsh SR. Perioperative fluid restriction in major abdominal surgery: systematic review and meta-analysis of randomized, clinical trials. World J Surg. 2013; 37:1193–202.

8. Gamlin F, Freeman J, Winslow L, Berridge J, Vucevic M. The haemodynamic effects of propofol in combination with ephedrine in elderly patients (ASA groups 3 and 4). Anaesth Intensive Care. 1999; 27:477–80.

9. Adsumelli RS, Steinberg ES, Schabel JE, Saunders TA, Poppers PJ. Sequential compression device with thigh-high sleeves supports mean arterial pressure during Caesarean section under spinal anaesthesia. Br J Anaesth. 2003; 91:695–8.

10. Kurukahvecioglu O, Sare M, Karamercan A, Gunaydin B, Anadol Z, Tezel E. Intermittent pneumatic sequential compression of the lower extremities restores the cerebral oxygen saturation during laparoscopic cholecystectomy. Surg Endosc. 2008; 22:907–11.

11. Kwak HJ, Lee JS, Lee DC, Kim HS, Kim JY. The effect of a sequential compression device on hemodynamics in arthroscopic shoulder surgery using beach-chair position. Arthroscopy. 2010; 26:729–33.

12. Kiefer N, Theis J, Putensen-Himmer G, Hoeft A, Zenker S. Peristaltic pneumatic compression of the legs reduces fluid demand and improves hemodynamic stability during surgery: a randomized, prospective study. Anesthesiology. 2011; 114:536–44.
13. Chen AH, Frangos SG, Kilaru S, Sumpio BE. Intermittent pneumatic compression devices -- physiological mechanisms of action. Eur J Vasc Endovasc Surg. 2001; 21:383–92.

14. Helmi M, Gommers D, Groeneveld AB. A review of the hemodynamic effects of external leg and lower body compression. Minerva Anestesiol. 2014; 80:355–65.
15. Sujata N, Arora D, Panigrahi BP, Hanjoora VM. A sequential compression mechanical pump to prevent hypotension during elective cesarean section under spinal anesthesia. Int J Obstet Anesth. 2012; 21:140–5.

16. Schwenk W, Böhm B, Fügener A, Müller JM. Intermittent pneumatic sequential compression (ISC) of the lower extremities prevents venous stasis during laparoscopic cholecystectomy. A prospective randomized study. Surg Endosc. 1998; 12:7–11.
17. Gaffney FA, Thal ER, Taylor WF, Bastian BC, Weigelt JA, Atkins JM, et al. Hemodynamic effects of Medical Anti-Shock Trousers (MAST garment). J Trauma. 1981; 21:931–7.

18. Janssen H, Treviño C, Williams D. Hemodynamic alterations in venous blood flow produced by external pneumatic compression. J Cardiovasc Surg (Torino). 1993; 34:441–7.
Fig. 1.
Subject flow diagram. Groups 1, 2, and 3 in each age patient represent control, sequential compression, and sustained compression of the lower extremities, respectively. Three and two patients were discontinued the intervention by severe hypotension or arrhythmia and the hemodynamic monitor or target-controlled infusion pump malfunctions in patients aged older than 65 years, respectively. In patients aged 65 years or younger, one patient was excluded due to over 70 in bispectral index with propofol 4 μg/ml and remifentanil 3 ng/ml of the effect-site concentration.
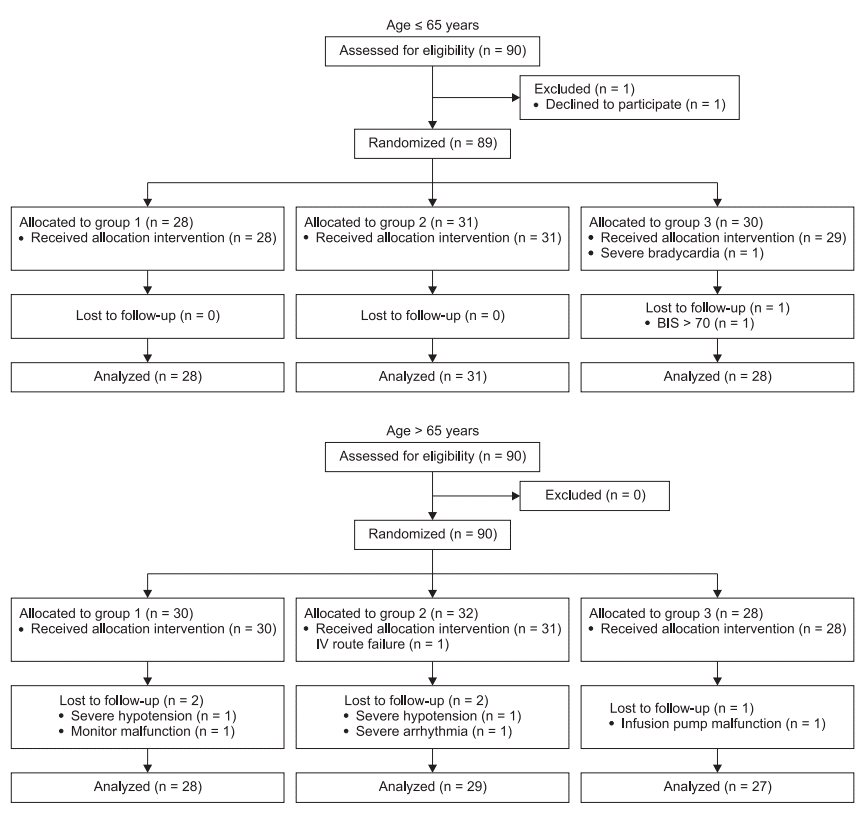
Fig. 2.
Patients treated with sustained pneumatic compression of the lower extremities (Group 3) had significantly higher mean arterial pressure (MAP) 1, 2, and 3 min after loss of consciousness (LOC) and 5 min after intubation (IT) compared with the control group (Group 1), but there were no differences between Group 1 and Group 2 (sequential compression) in patients aged 65 years or younger (A). However, differences were not observed among the three groups in patients older than 65 years (B). Pre-AN: preanesthetic baseline. *P < 0.05 compared with Group 1.
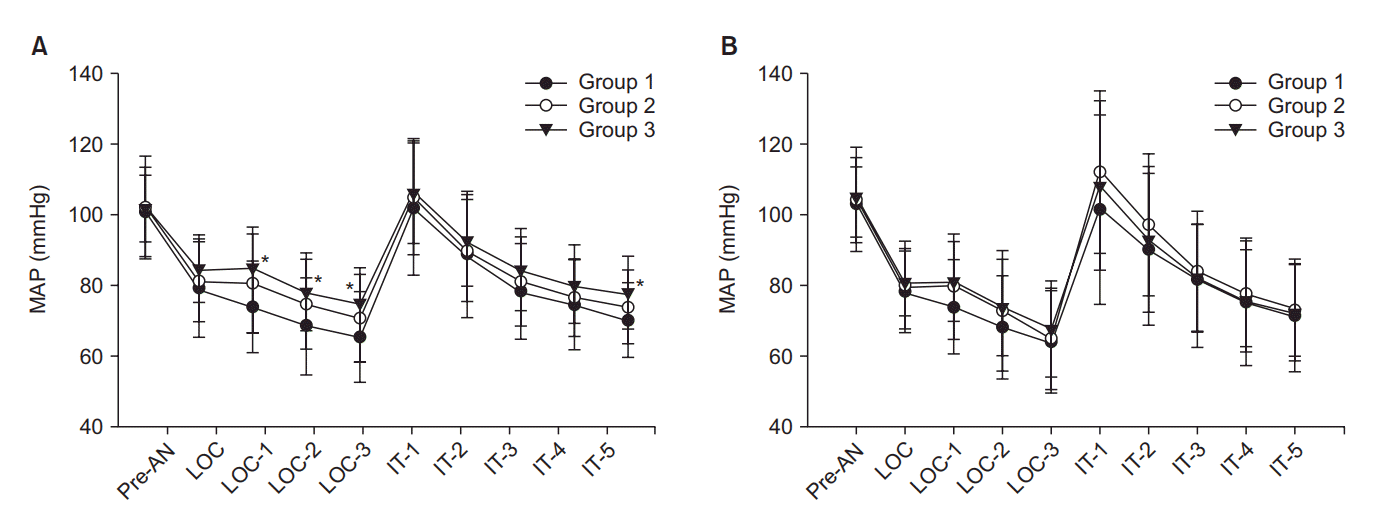
Fig. 3.
Cardiac index (CI) decreased after induction of anesthesia in the three groups. However, CI were significantly higher in Group 3 (sustained pneumatic compression) compared with Group 1 (control) 1, 2, and 3 min after loss of consciousness (LOC) in patients aged 65 years or younger (A). There was no difference in CI among the three groups in patients older than 65 years (B). Pre-AN: preanesthetic baseline, IT: intubation. *P < 0.05 compared with Group 1.
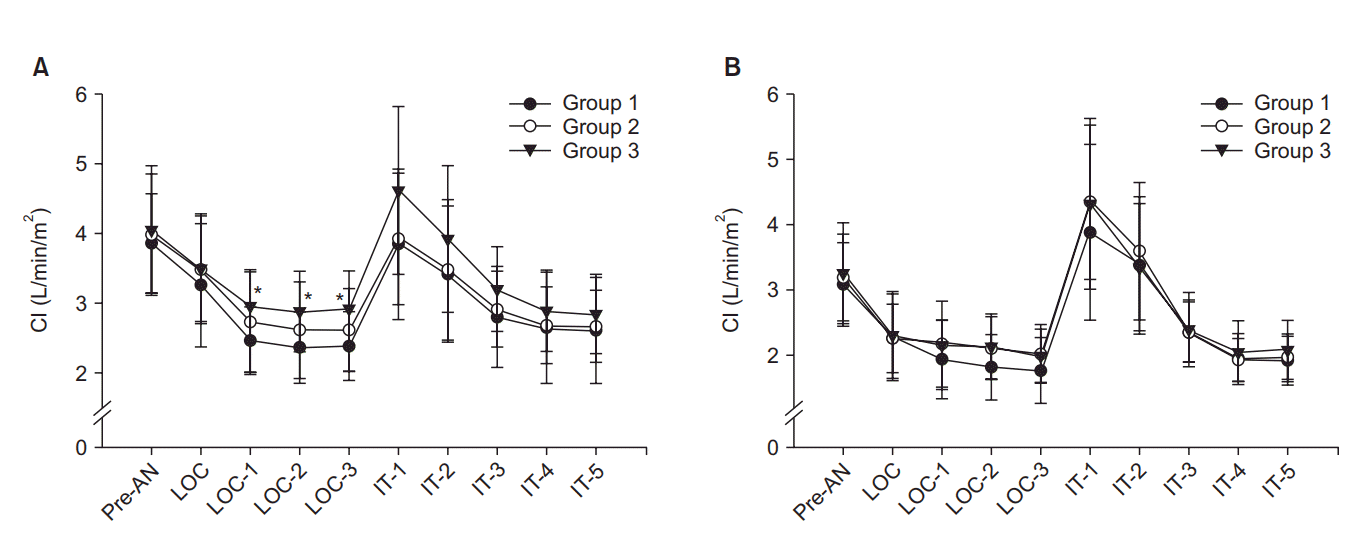
Fig. 4.
Stroke volume variation (SVV), a parameter of preload, was significantly lower in the sustained pneumatic compression group (Group 3) compared with the control (Group 1) after anesthesia induction, but there were no differences between Group 1 and Group 2 (sequential pneumatic compression) in patients aged 65 years or younger (A). Differences were not observed among the three groups in patients older than 65 years (B). Pre-AN: preanesthetic baseline, LOC: loss of consciousness, IT: intubation. *P < 0.05 compared with Group 1. †P < 0.05 compared with Group 2.
Table 1.
Patients Characteristics
Table 2.
Number of Patients Who Showed Hemodynamic Instability
|
Aged ≤ 65 years |
Aged > 65 years |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group 1 (n = 28) | Group 2 (n = 31) | Group 3 (n = 28) | P value | Group 1 (n = 28) | Group 2 (n = 29) | Group 3 (n = 27) | P value | ||
| MAP (mmHg) | < 60 | 9 | 6 | 1* | 0.022 | 11 | 11 | 10 | 0.955 |
| > 130 | 3 | 1 | 4 | 0.322 | 5 | 5 | 8 | 0.451 | |
| CI (L/min/m2) | < 2.2 | 8 | 8 | 4 | 0.401 | 20 | 17 | 14 | 0.318 |
| > 5.0 | 3 | 5 | 3 | 0.767 | 5 | 10 | 9 | 0.306 | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download