1. Webb CA, Mariano ER. Best multimodal analgesic protocol for total knee arthroplasty. Pain Manag. 2015; 5:185–96.

2. Kehlet H, Andersen LØ. Local infiltration analgesia in joint replacement: the evidence and recommendations for clinical practice. Acta Anaesthesiol Scand. 2011; 55:778–84.

3. Tripuraneni KR, Woolson ST, Giori NJ. Local infiltration analgesia in TKA patients reduces length of stay and postoperative pain scores. Orthopedics. 2011; 34:173.

4. Perlas A, Kirkham KR, Billing R, Tse C, Brull R, Gandhi R, et al. The impact of analgesic modality on early ambulation following total knee arthroplasty. Reg Anesth Pain Med. 2013; 38:334–9.

5. Kampitak W, Tanavalee A, Ngarmukos S, Amarase C, Apihansakorn R, Vorapalux P. Does adductor canal block have a synergistic effect with local infiltration analgesia for enhancing ambulation and improving analgesia after total knee arthroplasty? Knee Surg Relat Res. 2018; 30:133–41.

6. Steckelberg RC, Funck N, Kim TE, Walters TL, Lochbaum GM, Memtsoudis SG, et al. Adherence to a multimodal analgesic clinical pathway: a within-group comparison of staged bilateral knee arthroplasty patients. Reg Anesth Pain Med. 2017; 42:368–71.
7. Thobhani S, Scalercio L, Elliott CE, Nossaman BD, Thomas LC, Yuratich D, et al. Novel regional techniques for total knee arthroplasty promotereduced hospital length of stay: an analysis of 106 patients. Ochsner J. 2017; 17:233–8.
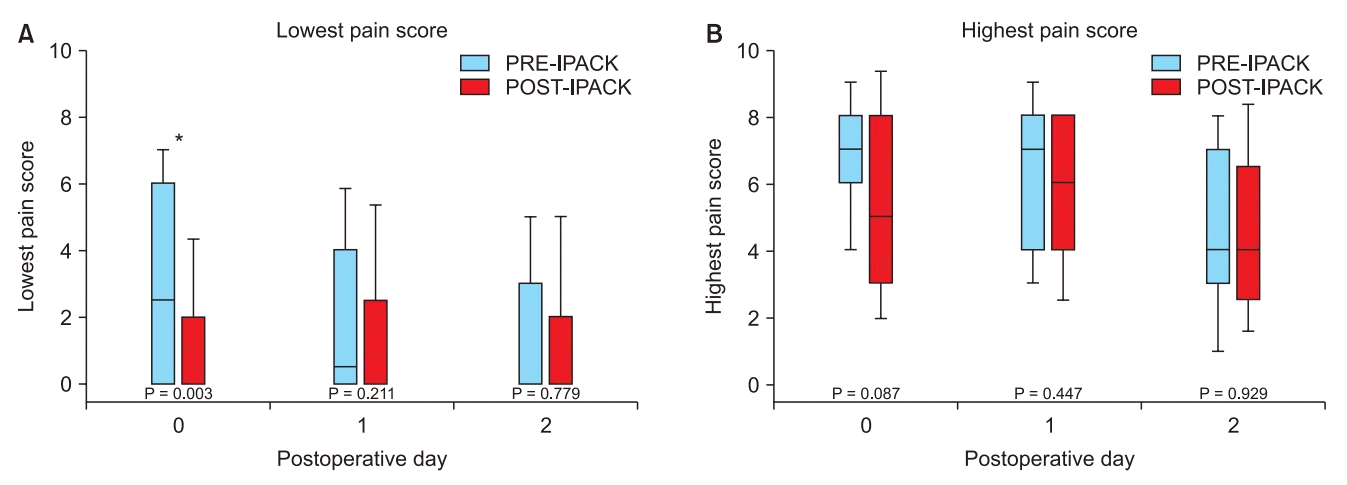
8. Kim DH, Beathe JC, Lin Y, YaDeau JT, Maalouf DB, Goytizolo E, et al. Addition of infiltration between the popliteal artery and the capsule of the posterior knee and adductor canal block to periarticular injection enhances postoperative pain control in total knee arthroplasty: a randomized controlled trial. Anesth Analg;2018. Advance Access published on Sep 12, 2018. doi: 10.1213/ANE.0000000000003794.
9. Walters TL, Howard SK, Kou A, Bertaccini EJ, Harrison TK, Kim TE, et al. Design and implementation of a perioperative surgical home at a veterans affairs hospital. Semin Cardiothorac Vasc Anesth. 2016; 20:133–40.

10. Mariano ER, Walters TL, Kim TE, Kain ZN. Why the perioperative surgical home makes sense for veterans affairs health care. Anesth Analg. 2015; 120:1163–6.

11. Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, Reed JE. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014; 23:290–8.

12. Mariano ER, Kim TE, Wagner MJ, Funck N, Harrison TK, Walters T, et al. A randomized comparison of proximal and distal ultrasoundguided adductor canal catheter insertion sites for knee arthroplasty. J Ultrasound Med. 2014; 33:1653–62.

13. Mudumbai SC, Walters TL, Howard SK, Kim TE, Lochbaum GM, Memtsoudis SG, et al. The Perioperative Surgical Home model facilitates change implementation in anesthetic technique within a clinical pathway for total knee arthroplasty. Healthc (Amst). 2016; 4:334–9.

14. Mudumbai SC, Auyong DB, Memtsoudis SG, Mariano ER. A pragmatic approach to evaluating new techniques in regional anesthesia and acute pain medicine. Pain Manag. 2018; 8:475–85.

15. Schwenk ES, Mariano ER. Designing the ideal perioperative pain management plan starts with multimodal analgesia. Korean J Anesthesiol. 2018; 71:345–52.

16. Memtsoudis SG, Poeran J, Zubizarreta N, Cozowicz C, Mörwald EE, Mariano ER, et al. Association of multimodal pain management strategies with perioperative outcomes and resource utilization: a population-based study. Anesthesiology. 2018; 128:891–902.
17. Sankineani SR, Reddy AR, Eachempati KK, Jangale A, Gurava Reddy AV. Comparison of adductor canal block and IPACK block (interspace between the popliteal artery and the capsule of the posterior knee) with adductor canal block alone after total knee arthroplasty: a prospective control trial on pain and knee function in immediate postoperative period. Eur J Orthop Surg Traumatol. 2018; 28:1391–5.

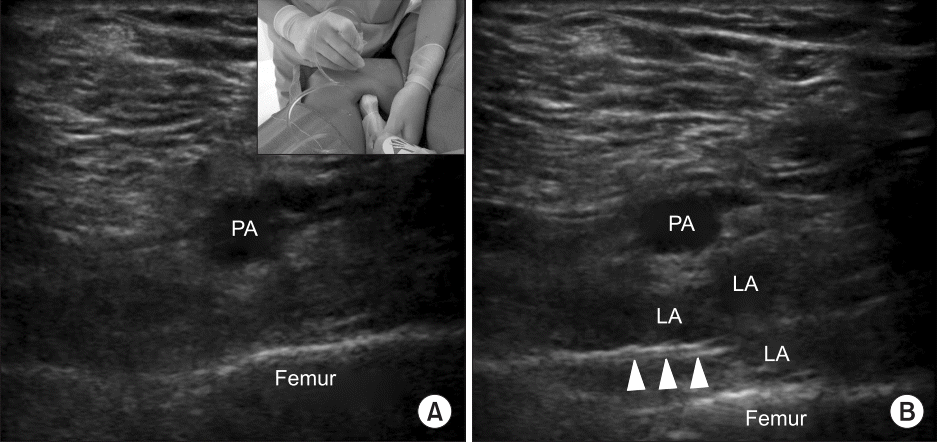
18. Niesen AD, Harris DJ, Johnson CS, Stoike DE, Smith HM, Jacob AK, et al. Interspace between popliteal artery and posterior capsule of the knee (IPACK) injectate spread: a cadaver study. J Ultrasound Med. 2019; 38:741–5.

19. Mudumbai SC, Kim TE, Howard SK, Workman JJ, Giori N, Woolson S, et al. Continuous adductor canal blocks are superior to continuous femoral nerve blocks in promoting early ambulation after TKA. Clin Orthop Relat Res. 2014; 472:1377–83.

20. Yajnik M, Hill JN, Hunter OO, Howard SK, Kim TE, Harrison TK, et al. Patient education and engagement in postoperative pain management decreases opioid use following knee replacement surgery. Patient Educ Couns. 2019; 102:383–7.

21. Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011; 104:510–20.

22. Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999; 282:1458–65.

23. Hill MV, Stucke RS, McMahon ML, Beeman JL, Barth RJ Jr. An educational intervention decreases opioid prescribing after generalsurgical operations. Ann Surg. 2018; 267:468–72.
24. Singh JA, Sloan J. Higher comorbidity, poor functional status and higher health care utilization in veterans with prevalent total knee arthroplasty or total hip arthroplasty. Clin Rheumatol. 2009; 28:1025–33.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download