Abstract
Background
Laparoscopic donor nephrectomy is considered less painful than open nephrectomy but is still associated with significant postoperative pain. Studies reported that intraperitoneal instillation of local anesthetics provides uncertain pain relief after laparoscopic surgery. This randomized, double-blind study evaluated the effect of intraperitoneal nebulization of ropivacaine on postoperative pain relief after laparoscopic donor nephrectomy.
Methods
Sixty patients undergoing elective laparoscopic donor nephrectomy were randomly assigned to receive either an instillation of 20 ml 0.5% ropivacaine after the induction of pneumoperitoneum or nebulization of 5 ml 1% ropivacaine before and after surgery. The primary outcome was the degree of pain relief (static and dynamic) after surgery. The secondary outcomes were postoperative fentanyl consumption, incidence of shoulder pain, unassisted walking and postoperative nausea and vomiting (PONV). Data were collected in the postanesthesia care unit (PACU) and at 6, 24, and 48 h after surgery.
Results
Compared to patients in the instillation group, those in the nebulization group showed significant reductions in postoperative pain and fentanyl consumption, and none complained of significant shoulder pain (visual analog scale score ≥ 30 mm). Within 20 h of surgery, 13.3% of patients in the instillation group and 93.3% in the nebulization group started unassisted walking (absolute risk reduction, 38%; P = 0.001). In the nebulization group, PONV was significantly reduced in the PACU and at 6 h.
Postoperative pain management is key to a patient’s early recovery. Patients undergoing laparoscopic donor nephrectomy experience moderate to severe pain, especially on the first postoperative day [1]. Laparoscopic procedures involve insufflation of the abdomen by using a gas, so that the endoscope can visualize the intra-abdominal contents without being in direct contact with the viscera or tissues and so that surgery can be performed using instruments introduced through additional ports. Laparoscopic donor nephrectomy is associated with postoperative pain due to the surgical incision, intra-abdominal tissue dissection/ trauma, and referred shoulder pain [2]. The laparoscopic surgical approach is associated with pain due to the intraperitoneal insufflation of carbon dioxide, which results in peritoneal stretching, diaphragmatic irritation, change in intra-abdominal pH, and retention of the insufflated gas in the abdominal cavity after surgery [3]. These factors may lead to visceral and shoulder pain due to the irritation of the peritoneal nerves.
Administration of intraperitoneal local anesthetics in laparoscopic surgeries as part of a multimodal approach to postoperative pain management is safe, but intraperitoneal instillation provides limited pain relief [4]. Failure to achieve adequate pain relief after intraperitoneal local anesthetic instillation may be attributed to the non-uniform distribution of local anesthetics throughout the peritoneal surface [5]. Intraperitoneal local anesthetic nebulization is a new technique of drug administration that should provide uniform dispersion of local anesthetic particles throughout the peritoneal cavity [6]. The analgesic effectiveness of this technique may depend upon the type of nebulization device and delivery mode. A microvibration-based nebulization device (Aeroneb ProⓇ system, Aerogen, Ireland) is superior to custom-made nebulization systems for ropivacaine delivery into the insufflation gas required to achieve pneumoperitoneum [7,8].
We hypothesized that pain relief after intraperitoneal ropivacaine nebulization would be superior to intraperitoneal ropivacaine instillation after laparoscopic donor nephrectomy. The aim of this clinical trial was to assess and compare the analgesic efficacy of intraperitoneal ropivacaine nebulization with the Aeroneb ProⓇ device to that of intraperitoneal ropivacaine instillation in laparoscopic donor nephrectomy.
This single-center, randomized, parallel-group, double-blind study was approved by the ethics committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India (Ref PGI/BE/38/2013), and written informed consent was obtained from all patients participating in the trial. The trial was registered at the Clinical Trials Registry - India (CTRI/2014/08/004926; Principal investigator: Anil Agarwal; Date of registration: 25/08/2014). We enrolled 60 patients of either sex, aged 18–65 years, with American Society of Anesthesiologists physical status I–II, and scheduled them to undergo elective laparoscopic donor nephrectomy. Patients were excluded if they had been using analgesic drugs before surgery or posted for emergency or urgent surgery; had cognitive impairment or mental retardation, a history of seizures or chronic therapy with antiepileptic drugs, severe hepatic or renal impairment, allergy to one of the specific drugs under study, acute infection or chronic disease, a history of alcohol or drug addiction, were pregnant or lactating or patient’s inability to convey level of pain due to language barrier or converted to open nephrectomy.
On the day of surgery, a research assistant not involved in patient care confirmed patient eligibility, obtained written consent, and gave the sealed envelope containing the patient’s allocation and instruction for solution preparation to a trained anesthesia technician who was also not involved in the study. The solutions were prepared in 20-ml transparent syringes containing 20 ml of 0.5% ropivacaine or 20 ml of normal saline and two 5-ml transparent syringes containing 5 ml of 1% ropivacaine or 5 ml of normal saline according to the randomization sequence in a blinded fashion. In case of an emergency related to the study or study drugs, the anesthesia technician was authorized to disclose the contents of the syringe to the case anesthesiologist who was not involved in the study and to the research assistant. Patients were randomized using a computer-generated sequence to receive either intraperitoneal instillation of ropivacaine or intraperitoneal nebulization of ropivacaine. Patients in the instillation group received intraperitoneal instillation of 20 ml (100 mg) of 0.5% ropivacaine, after the induction of pneumoperitoneum but before kidney dissection, plus intraperitoneal nebulization of 5 ml normal saline, before the start of kidney dissection and again at the end of surgery just before the deflation of pneumoperitoneum. Patients in the nebulization group received intraperitoneal nebulization of 5 ml (50 mg) of 1% ropivacaine, before the start of kidney dissection and again at the end of surgery just before the deflation of pneumoperitoneum, plus intraperitoneal instillation of 20 ml normal saline after the induction of pneumoperitoneum but before the start of kidney dissection.
Intraperitoneal nebulization of ropivacaine or saline was performed using the Aeroneb ProⓇ device, which was placed in series between the insufflator and insufflation tubing. Ropivacaine or normal saline was carried to the peritoneal cavity by the insufflation gas through a 200-cm-long insufflator tubing connected to the umbilical port. The first nebulization was initiated simultaneously with gas insufflation through the umbilical port, while the other ports were being inserted. The second nebulization was performed at the end of surgery, just before the withdrawal of the ports. Nebulization was terminated once the nebulizer chamber was empty, and the average time was 5–8 min. The pharmacokinetic profile of ropivacaine was similar in both instillation and nebulization groups, except for the lower absorption constant in nebulization group.
Laparoscopic live donor nephrectomy was performed according to standard surgical protocols. The surgery was performed via the left transperitoneal approach. The donor was placed in the modified lateral decubitus position. Pneumoperitoneum was achieved by insufflations of non-humidified and non-heated carbon dioxide at an intraperitoneal pressure of 12–14 mmHg. The surgical technique consisted of one 12-mm port placed at the umbilicus, another 12-mm port between the umbilicus and anterior superior iliac spine (spinoumbilical port), a 5-mm port approximately 3 cm below the costal margin and 3 cm lateral to the midline, and a fourth 5-mm port 4 cm below the costal margin in the anterior axillary line, if needed. The graft was gently retrieved through the 5-cm iliac fossa muscle splitting incision, an oblique extension of the spinoumbilical port.
The anesthetic technique was standardized for each patient. All patients were premedicated with lorazepam (1.0 mg per oral [PO]) and ranitidine (150 mg PO) the night before surgery, and ranitidine (150 mg PO) with a sip of water was repeated 2 h before being shifted for surgery. On arrival in the operation room, standard monitoring was performed, and midazolam (0.05–0.07 mg/kg IV) and fentanyl (2–5 μg/kg intravenous [IV]) were administered before the induction of anesthesia. General anesthesia was induced using propofol (2–3 mg/kg IV), and muscle relaxation was achieved using atracurium (0.5–0.6 mg/kg IV) to facilitate endotracheal intubation. Anesthesia was maintained using sevoflurane (1.5%–2.5% end-tidal concentration), intermittent fentanyl boluses (1–2 μg/kg titrated to maintain noninvasive mean arterial blood pressure and heart rate + 20% of the baseline value), and intermittent atracurium (0.1 mg/kg titrated to maintain a train-of-four (TOF) count of 1 or 2, and according to clinical needs). Patients were mechanically ventilated with a constant flow and I : E ratio of 1 : 2; the respiratory rate and tidal volume were adjusted to maintain an end-tidal carbon dioxide pressure of 35–40 mmHg and plateau pressure below 30 cmH2O. Perioperative temperature was monitored using an esophageal temperature probe, and patients were kept warm by using warmers as well as warmed intravenous fluids. At the end of the surgical procedure, residual muscle paralysis was reversed using neostigmine (0.05 mg/kg IV) and glycopyrrolate (10 μg/ kg IV), and patients were extubated once the clinical and TOF criteria were achieved. For postoperative nausea and vomiting (PONV) prophylaxis, 4 mg dexamethasone after the induction of anesthesia and 4 mg ondansetron at the end of surgery were administered intravenously to all patients.
All patients received paracetamol (15 mg/kg IV) infused over 15 min before the start of surgery and postoperatively every 6 h for 48 h. At the end of surgery, each portal site was infiltrated with 3 ml of 0.03% ropivacaine and the incision site with 5 ml of 0.03% ropivacaine. All patients received fentanyl-based intravenous patient-controlled analgesia (IV-PCA) in the postanesthesia care unit (PACU). The PCA pump was programmed with a demand dose of 30 μg fentanyl, with a lockout interval of 10 min and a maximum of 3 doses/h. Postoperatively, all patients were encouraged to ambulate as early as possible. As per our routine practice, all patients were hospitalized for up to 72 h after surgery.
Patient age, sex, body weight, body mass index (BMI), intraoperative opioid use, duration of surgery, residual volume of local anesthetic in the nebulization unit, and sign of local anesthetic toxicity (e.g., unexplained hypotension, intraoperative arrhythmias, and unexplained delayed awakening) were recorded. Patient temperature during surgery and in the PACU and the length of PACU stay were also recorded. The postoperative pain intensity at rest (static pain) and on deep breathing, coughing, or movement (dynamic pain) were assessed using the visual analog scale (VAS) pain scoring system (0 mm = no pain and 100 mm = worst possible pain); the incidence of significant shoulder pain (VAS score ≥ 30 mm), cumulative fentanyl consumption, time to unassisted walking, and incidence of PONV were also recorded. All data were collected by a dedicated research assistant in the PACU and at 6, 24, and 48 h after surgery. The research assistants involved in data collection as well as the nurses and doctors involved in patient care were unaware of the study group assignment.
The primary outcome was pain intensity (static and dynamic) after surgery, measured using the VAS scoring system. Sample size calculations were based on previous studies involving intraperitoneal local anesthetic nebulization and instillation after elective laparoscopic surgery [5,6]. A pilot study conducted at our institution showed that the mean reduction in static pain in the nebulization group was 41 mm ± 14 mm, while that in the instillation group was 28 mm ± 14 mm. We considered a 30% reduction in pain intensity as a significant change. To attain a power of 90% (1-β) and a significance level of 0.05 (α error), we needed to enroll 27 patients in each group to reject the null hypothesis. We enrolled 30 patients in each group to account for dropouts if any.
This study evaluated the effect of intraperitoneal nebulization of ropivacaine on pain intensity after laparoscopic donor nephrectomy. The secondary outcomes were postoperative fentanyl consumption, incidence of shoulder pain, unassisted walking and PONV.
Continuous variables, including age, body weight, BMI, temperature, intraoperative and postoperative fentanyl consumption, duration of surgery, length of PACU stay, static and dynamic pain scores, and time to unassisted walking, were presented as mean ± standard deviation (SD) and 95% CI and were compared using two-sided Student’s t test or the Mann–Whitney U test as appropriate. Discrete variables, including the number of patients of a particular sex, number of patients with significant postoperative pain (dynamic VAS score ≥ 30 mm), patients with significant shoulder pain, proportion of patients walking without assistance within 20 h after surgery, and patients with PONV, were presented as frequency and 95% CI, as well as absolute risk reduction. These were analyzed using the chi-square test or Fisher’s exact test as appropriate. Repeated-measures analysis of variance (ANOVA) was carried out to determine the effect of time and time-to-group interaction on the change in VAS scores and fentanyl consumption.
Effect size is the magnitude of the quantitative relationship between one variable (e.g., a variable that defines a treatment group) and another variable (e.g., a specific outcome). Effect size can be measured as the difference between two means divided by the SD of the two conditions, and is used to interpret changes in the health status. To compare the relative impact of ropivacaine nebulization on the intensity of postoperative pain, fentanyl consumption, length of PACU stay, and unassisted walking time, the effect size of ropivacaine nebulization was calculated using Cohen’s d test. Cohen classified effect sizes as small (d = 0.2), medium (d = 0.5), and large (d = 0.8).
All statistical comparisons were performed using IBM SPSS Statistics for Windows/Macintosh, Version 20.0 (IBM Corp., USA). A P value ≤ 0.05 was considered significant.
Sixty patients were included in the data analysis (Fig. 1). No patients dropped out from the study. No significant differences existed between the groups with respect to age, body weight, BMI, sex, duration of surgery, and temperature after surgery (Table 1). A significant difference in intraoperative fentanyl use (Cohen’s d = 1.33 ‘large effect’; P = 0.001) and length of PACU stay (Cohen’s d = 2.2 ‘large effect’; P = 0.002) were observed between the groups (Table 1).
Significant differences were also observed between the groups with respect to the static and dynamic pain scores in the PACU and at 6 h after surgery (P = 0.001). At 24 h after surgery, no significant difference was observed between the groups in the static pain score (Cohen’s d = 0.39 ‘small effect’; P = 0.13); however, a significant difference was reported in the dynamic pain score (Cohen’s d = 2.07 ‘large effect’; P = 0.002). No significant differences were observed between the groups with respect to the static and dynamic pain scores at 48 h after surgery and at the time of discharge. The repeated-measures ANOVA revealed a significant effect of time (P < 0.001) and time-to-group interaction (P < 0.001) in the change in static and dynamic pain (Table 2).
Significant postoperative pain (dynamic VAS score ≥ 30 mm) was equal in both the groups in the PACU. At 6 h, only 1 patient in the nebulization group had a dynamic VAS score < 30 mm; however, at 24 h, VAS scores ≥ 30 mm were observed in 7 patients in the nebulization group and 26 patients in the instillation group. No significant pain relief (dynamic VAS score < 30 mm) was observed after ropivacaine nebulization in the PACU and at 6 h (absolute risk reduction at 6 h was 0.85% and at 24 h was 28%) (Table 3).
Significant shoulder pain was not observed in the nebulization group, but was observed in 22 (73%) patients in the instillation group (absolute risk reduction, 50%; P = 0.001); this also resulted in a significant reduction in postoperative fentanyl consumption. Over the 48-h postoperative period, the nebulization group consumed 791 ± 97 μg fentanyl whereas the instillation group consumed 1182 ± 73 μg (Cohen’s d = 3.68 ‘large effect’; P = 0.002). Unassisted walking was earlier in the nebulization group than in the instillation group. The mean time to unassisted walking after surgery was 22.03 ± 1.47 h in the instillation group and 18.20 ± 1.95 h in the nebulization group (Cohen’s d = 2.21 ‘large effect’; P = 0.001). Within 20 h of surgery, 13.3% of patients in the instillation group and 93.3% of those in the nebulization group started unassisted walking (absolute risk reduction, 38%; P = 0.001). The repeated-measures ANOVA revealed significant effect of time (P < 0.001) and time-to-group interaction (P < 0.001) in the change in fentanyl consumption.
No significant difference was observed between the groups with respect to the length of hospital stay. Patients were discharged 3.08 ± 0.27 days after surgery in the instillation group and at 3.00 ± 0.29 days after surgery in the nebulization group (Cohen’s d = 0.28 ‘small effect’; P = 0.25).
Significant differences were observed in the proportion of patients with PONV. While 10 (33%) patients in the instillation group complained of nausea in the PACU, only 3 (10%) in the nebulization group had similar complaints (absolute risk reduction, 26%; P = 0.03). At 6 h after surgery, 5 (17%) patients in the nebulization group and 16 (53%) in the instillation group complained of nausea; moreover, 3 (10%) patients in the instillation group had one episode of vomiting (absolute risk reduction, 29%; P = 0.01). At 24 h after surgery, 5 (17%) patients complained of nausea and 1 (3%) patient vomited in the instillation group, whereas no patients in the nebulization group had similar problems (absolute risk reduction, 50%; P = 0.04). Local anesthetic toxicity was not reported in any patient during or after surgery, in either group.
In this first-ever study, we compared intraperitoneal nebulization of ropivacaine with intraperitoneal instillation of ropivacaine for postoperative pain relief in a patient population undergoing donor nephrectomy. We observed that intraperitoneal ropivacaine nebulization significantly reduced postoperative pain (static and dynamic), referred shoulder pain, fentanyl consumption, time to unassisted walking, and PONV after laparoscopic donor nephrectomy.
The pharmacokinetic profile of ropivacaine was similar in both the groups, except for the lower absorption constant in the nebulization group. The absorption of ropivacaine in the nebulization group may have been affected by the longer duration of drug administration, pneumoperitoneum-induced capillary compression, ropivacaine-induced vasoconstriction, and local dilution of ropivacaine on the contact surface layer by the intraperitoneal fluids, which may modify the plasma Cmax and Tmax [9].
Conventional methods of providing analgesia for laparoscopic procedures include parenteral nonsteroidal anti-inflammatory drugs and opioids. Although intraoperative sympathetic stimulation, which occurs because of pneumoperitoneum, best responds to opioids, it leads to undesired side effects such as delayed recovery and obstructed breathing in the immediate postoperative period. Opioids are also inadequate to treat the postoperative dynamic pain in laparoscopic donor nephrectomy. Thus, the multimodal analgesic methods that supplement analgesia with regional anesthesia techniques have been reported to control the dynamic pain in laparoscopic donor nephrectomy, as well as to drastically reduce the consumption of opioids, thereby reducing their undesired side effects [10,11]. Regional techniques aimed at controlling peritoneal stretch pain require the deposition of local anesthetic in the peritoneal cavity, mainly on the parietal peritoneum that is pain sensitive. Continuous infusion of ropivacaine in laparoscopic donor nephrectomy provided a significant reduction in postoperative pain, morphine consumption, bowel recovery, and the length of hospital stay, but was associated with technical issues related to catheter placement and postoperative catheter dislodgement [12–14].
Reduction in intraoperative fentanyl consumption and the length of PACU stay in the nebulization group in the present study was more significant than previously reported [5]. At 6 h after surgery, we observed a significant reduction in the VAS scores for both static and dynamic pain in the nebulization group, but at 24 h, only the dynamic VAS score was significantly reduced in the nebulization group. Postoperative fentanyl consumption was significantly reduced at all time intervals in the nebulization group. Similar observations were also made by Ingelmo et al. [15] and Bhatia et al. [16], but Bucciero et al. [5] made different observations. Scalia Catenacci et al. [17] studied the effect of intraperitoneal nebulization of ropivacaine in laparoscopic ovarian cyst resection and observed similar results, except that the nebulization group had a higher incidence of shivering. Although a similar technique of postoperative pain relief has been applied in laparoscopic cholecystectomy and laparoscopic gynecological surgeries with variable results, intraperitoneal nebulization of ropivacaine has not been reported to date for donor nephrectomy; moreover, the duration of surgery was significantly longer in our study.
The reduced shoulder pain observed in our study was also reported in previous studies [5,8]. The lower incidence of shoulder pain in the nebulization group may be due to the uniform spread of ropivacaine along with the humidified insufflation gas throughout the peritoneum, including the area under the diaphragm. The reduced incidence of shoulder pain may also have allowed earlier ambulation.
The overall increased incidence of PONV in this study (26.67% at PACU, 35% at 6 hours and 10% at 24 hours), unlike in previous studies [5,15], was possibly because of the inclusion of a large number of female patients, duration of surgery over 60 min, inclusion of nonsmokers, and postoperative opioid consumption. However, the incidence of PONV was significantly lower in the nebulization group than in previously studied groups [5,15]. The significantly reduced incidence of PONV in nebulization group compared to the instillation group may be attributed to lower fentanyl requirement and reduced pain in the former group.
Intraperitoneal nebulization of ropivacaine is claimed to be effective in most studies, but the study by Kaufman et al. [18] on laparoscopic gynecologic surgeries showed no significant benefit of this technique, possibly because of their small sample size. Another study on laparoscopic appendectomy conducted in a pediatric population by Baird et al. [19] showed no difference in morphine consumption and postoperative pain score, possibly because pain perception in the pediatric population was completely different from that in an adult population.
In this study, we used a commercially available high-frequency vibrating-mesh nebulizer, which is reusable and easy to assemble. It also allows simultaneous and efficient delivery of the local anesthetic while the surgical procedure is being performed [7]. The particle size generated by the Aeroneb ProⓇ device is smaller than 5 µm; thus, it can be presumed that the local anesthetic spread uniformly throughout the peritoneal surface [20]. The probable mechanism of action of the local anesthetic delivered via nebulization is its effect on the peritoneal nerve endings, which are responsible for local and systemic modulation of the inflammatory process.
We did not observe any symptoms related to local anesthetic toxicity. Ropivacaine having a significantly higher threshold for cardio-toxicity and CNS-toxicity than bupivacaine due to its stereo-selective properties [21]. The total amount of ropivacaine used in this study was far below the maximum dose for infiltration anesthesia in an adult patient [22]. The pharmacokinetics of ropivacaine was described in an animal study, which showed that 3 mg/kg of nebulized ropivacaine was similar to instilled ropivacaine [9]. A recent study compared the effects and pharmacokinetics of intraperitoneal nebulization of different doses (50 mg, 100 mg, and 150 mg) of ropivacaine by using the Aeroneb ProⓇ system in patients undergoing laparoscopic cholecystectomy, and found that the absorption of ropivacaine and its pharmacokinetic profile were independent of its dose. The plasma concentration remained consistent under the mean toxic plasma concentration. They also found that increasing the dose of ropivacaine from 50 mg to 150 mg did not reduce postoperative pain intensity, morphine consumption, incidence of PONV, or readiness for discharge, but was instead associated with the incidence of postoperative shivering [23].
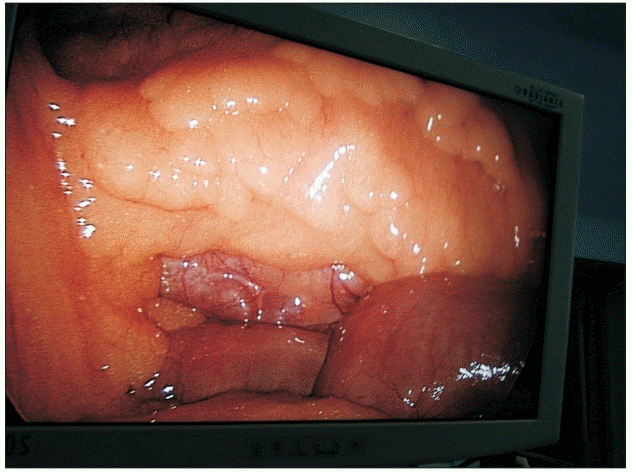
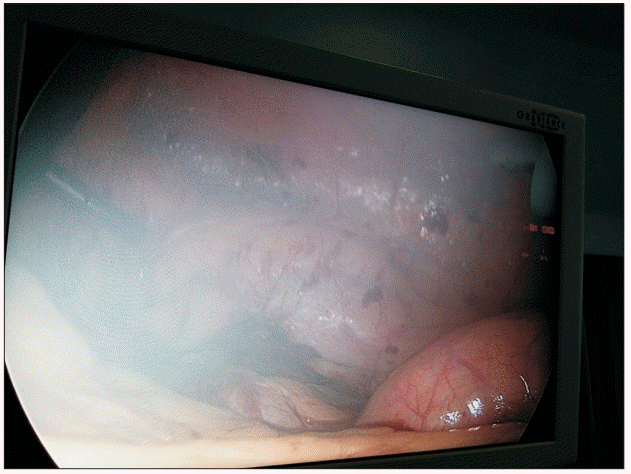
One of the limitations of nebulization is that the small-sized droplets create a ‘foggy’ environment in the surgical field, which interferes with the surgeon’s field of vision (Figs. 2 and 3). The higher the local anesthetic concentration, the lower the nebulization time and consequently lesser the ‘fog’ in the surgical field; therefore, we used 1% ropivacaine in the nebulization group and divided it into two doses (the total dose took on average 14–16 min to administer). The initial nebulization was performed through the central port during the insertion of the other ports, and the second nebulization was performed just before the exsufflation of pneumoperitoneum. The other limitation of this study is the lack of a control group. The decision not to have a control group was based on the findings of previous studies, which demonstrated limited or no benefit of intraperitoneal instillation. The study conducted by Alkhamesi et al. [8] did not find any difference between their local anesthetic instillation group and the control group.
Another criticism against this study may be the use of different concentrations and volumes of ropivacaine in the instillation and nebulization groups. The concentration (0.5%) and volume (20 ml) of ropivacaine in the instillation group was based on standard practice, and the lower volume (10 ml) and higher concentration (1%) of ropivacaine in the nebulization group was based on nebulization time (the Aeroneb ProⓇ device can deliver 5 ml of solution in 5–8 min). Moreover, a recent study showed that increasing the dose of ropivacaine from 50 mg to 150 mg did not show any clinical benefit [23].
Patients undergoing laparoscopic donor nephrectomy remained in the hospital for 3 days owing to our institutional protocol. Therefore, we could not assess the potential benefits of early discharge in the nebulization group.
In conclusion, intraperitoneal ropivacaine nebulization provides better postoperative pain control (static and dynamic) and reduces referred shoulder pain, fentanyl consumption, time to unassisted walking, and PONV in patients undergoing laparoscopic donor nephrectomy. We therefore suggest that intraperitoneal nebulization of ropivacaine may be used as a routine procedure in all patients undergoing laparoscopic donor nephrectomy. Future studies should aim to develop devices that enable faster nebulization closer to the umbilical port, without creating a foggy environment during the procedure.
Notes
References
1. Gorevski E, Wead S, Tevar A, Succop P, Volek P, Martin-Boone J. Retrospective evaluation of donor pain and pain management after laprascopic nephrectomy. Transplant Proc. 2011; 43:2487–91.

2. Ergün M, Berkers AW, van der Jagt MF, Langenhuijsen JF, van Özdemir-Brunschot D, van der Vliet JA, et al. Components of pain assessment after laparoscopic donor nephrectomy. Acta Anaesthesiol Scand. 2014; 58:219–22.

3. Mouton WG, Bessell JR, Otten KT, Maddern GJ. Pain after laparoscopy. Surg Endosc. 1999; 13:445–8.

4. Boddy AP, Mehta S, Rhodes M. The effect of intraperitoneal local anesthesia in laparoscopic cholecystectomy: a systematic review and meta-analysis. Anesth Analg. 2006; 103:682–8.

5. Bucciero M, Ingelmo PM, Fumagalli R, Noll E, Garbagnati A, Somaini M, et al. Intraperitoneal ropivacaine nebulization for pain management after laparoscopic cholecystectomy: a comparison with intraperitoneal instillation. Anesth Analg. 2011; 113:1266–71.
6. Alkhamesi NA, Ridgway PF, Ramwell A, McCullough PW, Peck DH, Darzi AW. Peritoneal nebulizer: a novel technique for delivering intraperitoneal therapeutics in laparoscopic surgery to prevent locoregional recurrence. Surg Endosc. 2005; 19:1142–6.

7. Greib N, Schlotterbeck H, Dow WA, Joshi GP, Geny B, Diemunsch PA. An evaluation of gas humidifying devices as a means of intraperitoneal local anesthetic administration for laparoscopic surgery. Anesth Analg. 2008; 107:549–51.

8. Alkhamesi NA, Peck DH, Lomax D, Darzi AW. Intraperitoneal aerosolization of bupivacaine reduces postoperative pain in laparoscopic surgery: a randomized prospective controlled double-blinded clinical trial. Surg Endosc. 2007; 21:602–6.

9. Betton D, Greib N, Schlotterbeck H, Joshi GP, Ubeaud-Sequier G, Diemunsch P. The pharmacokinetics of ropivacaine after intraperitoneal administration: instillation versus nebulization. Anesth Analg. 2010; 111:1140–5.
10. Güner Can M, Göz R, Berber İ, Kaspar Ç, Çakır Ü. Ultrasound/laparoscopic camera-guided transversus abdominis plane block for renal transplant donors: a randomized controlled trial. Ann Transplant. 2015; 20:418–23.

11. Myhre M, Romundstad L, Stubhaug A. Pregabalin reduces opioid consumption and hyperalgesia but not pain intensity after laparoscopic donor nephrectomy. Acta Anaesthesiol Scand. 2017; 61:1314–24.

12. Panaro F, Gheza F, Piardi T, Woehl Jaegle ML, Audet M, Cantù M, et al. Continuous infusion of local anesthesia after living donor nephrectomy: a comparative analysis. Transplant Proc. 2011; 43:985–7.

13. Yoost TR, McIntyre M, Savage SJ. Continuous infusion of local anesthetic decreases narcotic use and length of hospitalization after laparoscopic renal surgery. J Endourol. 2009; 23:623–6.

14. Biglarnia AR, Tufveson G, Lorant T, Lennmyr F, Wadström J. Efficacy and safety of continuous local infusion of ropivacaine after retroperitoneoscopic live donor nephrectomy. Am J Transplant. 2011; 11:93–100.

15. Ingelmo PM, Bucciero M, Somaini M, Sahillioglu E, Garbagnati A, Charton A, et al. Intraperitoneal nebulization of ropivacaine for pain control after laparoscopic cholecystectomy: a double-blind, randomized, placebo-controlled trial. Br J Anaesth. 2013; 110:800–6.

16. Bhatia N, Mehta S, Saini V, Ghai B, Kaman L. Comparison of intraperitoneal nebulization of ropivacaine with ropivacaine-fentanyl combination for pain control followinglaparoscopic cholecystectomy: a randomized, double-blind, placebo-controlled trial. J Laparoendosc Adv Surg Tech A. 2018; 28:839–44.
17. Scalia Catenacci S, Lovisari F, Peng S, Allegri M, Somaini M, Ghislanzoni L, et al. Postoperative analgesia after laparoscopic ovarian cyst resection: double-blind multicenter randomized control trial comparing intraperitoneal nebulization and peritoneal instillation of ropivacaine. J Minim Invasive Gynecol. 2015; 22:759–66.

18. Kaufman Y, Hirsch I, Ostrovsky L, Klein O, Shnaider I, Khoury E, et al. Pain relief by continuous intraperitoneal nebulization of ropivacaine during gynecologic laparoscopic surgery--a randomized study and review of the literature. J Minim Invasive Gynecol. 2008; 15:554–8.

19. Baird R, Ingelmo P, Wei A, Meghani Y, Perez EV, Pelletier H, et al. Nebulized analgesia during laparoscopic appendectomy (NALA): A randomized triple-blind placebo controlled trial. J Pediatr Surg. 2019; 54:33–8.

20. Bauer A, McGlynn P, Bovet LL, Mims PL, Curry LA, Hanrahan JP. Output and aerosol properties of 5 nebulizer/compressor systems with arformoterol inhalation solution. Respir Care. 2009; 54:1342–7.
21. Knudsen K, Beckman Suurküla M, Blomberg S, Sjövall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997; 78:507–14.

22. Berde C, Strichartz G. In: Miller’s Anesthesia. 8th ed. In : Miller RD, Cohen NH, Eriksson LI, Fleisher LA, WienerKronish JP, Young WL, editors. Philadelphia, Churchill Livingstone: Elsevier;2015. p. 1028–55.
23. Allegri M, Ornaghi M, Ferland CE, Bugada D, Meghani Y, Calcinati S, et al. Peritoneal nebulization of ropivacaine during laparoscopiccholecystectomy: dose finding and pharmacokinetic study. Pain Res Manag. 2017; 2017:4260702.
Table 1.
Patient Characteristics
Table 2.
Postoperative Pain Scores (VAS) at Different Postoperative Time Points
Table 3.
Postoperative Cumulative Fentanyl Consumption in Micrograms




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download