This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The breast is innervated by the intercostal nerves and the brachial plexus. We propose a technique to perform breast surgery without general anesthesia using the erector spinae plane (ESP) block and selective block of four nerves that arise from the brachial plexus innervate the breast and the axilla (SBP block).
Case
A 77-year-old man with breast cancer was scheduled for radical mastectomy and axillary clearance. He had a previous history of myocardial infarction with dilated cardiomyopathy and severely impaired ejection fraction. The surgery was performed under regional anesthesia with combined ESP and SBP block. The patient did not require opioids or other supplemental analgesics intra- or postoperatively and was discharged uneventfully.
Conclusions
SBP is a novel block that selectively blocks branches of the brachial plexus that innervate the breast.
Go to :

Keywords: Brachial plexus, Breast surgery, Erector spinae plane block, Regional anesthesia
Erector spinae plane (ESP) block, first described by Forero et al. [
1] in 2016, is a fascial block wherein the local anesthetic is injected into the plane between the erector spinae muscle and the transverse process of the underlying vertebra. ESP block was described for analgesia during breast surgery [
2], and as an anesthetic technique [
3,
4].
Kimachi et al. [
3] proposed ESP block as the sole technique to provide anesthesia to the breast and axillary regions while De Cassai et al. [
4] proposed combining ESP with pectoral nerve (PECS) block.
In this case report, we propose a third approach based on ESP and selective brachial plexus (SBP) blocks. Using this approach, we aim to block the medial pectoral (MPN), the lateral pectoral (LPN), the long thoracic (LTN), and the thoracodorsal (TDN) nerves that arise from the brachial plexus and innervate the breast and axillary region. We present the first case report of this technique and discuss in detail the SBP block and its limitations.
Case Report
A 77-year-old man, who was 172 cm tall and weighed 70 kg, was scheduled for radical mastectomy and axillary clearance for breast cancer. He had a history of myocardial infarction one year previously, for which he had undergone insertion of multiple bare metal coronary artery stents. He had developed post-infarction dilated cardiomyopathy with a severe reduction in ejection fraction (30%), a marked increase in the left ventricular diastolic diameter (6.8 cm), and global left ventricular hypokinesia with apical akinesia. He also had moderate mitral and minimal tricuspid valve insufficiency. His preoperative chest radiograph revealed cardiomegaly. His past medical history also included hypertension, diagnosed forty years ago. He was also a current, light smoker (less than 10 cigarettes per day). At pre-anesthetic evaluation, he was on dual antiplatelet therapy with aspirin and clopidogrel and on valsartan for hypertension. After specialist consultation, we decided to continue preoperative medications.
We planned a regional anesthesia technique using a combination of ESP and SBP block to avoid the use of general anesthesia. This technique was explained to the patient, and a written informed consent was obtained for the procedure.
We used a high-frequency linear ultrasound transducer (M-Turbo, FUJIFILM SonoSite Europe, The Netherlands) and 22 G × 50 mm needles (SonoPlex STIM, Pajunk, Germany) for the procedure.
The ESP block was performed as described by Forero et al. [
1] with 20 ml of 0.5% ropivacaine. The patient was placed in the supine position with the ipsilateral arm abducted to perform the SBP block. The LTN was identified at the C7 level within the middle scalene muscle; 3 ml of 0.5% ropivacaine was injected to perform the block (
Fig. 1). The TDN was identified in the infraclavicular region near the thoracodorsal artery and blocked with 3 ml of 0.5% ropivacaine (
Fig. 2C). The LPN and the MPN were blocked by injection of 10 ml of 0.5% ropivacaine between the pectoralis major and pectoralis minor muscles (
Fig. 3). A total dose of 36 ml of 0.5% ropivacaine was used to perform the blocks.
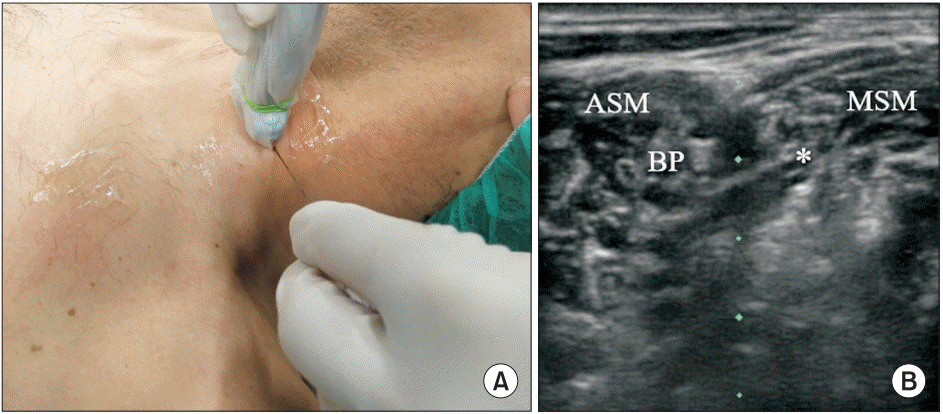 | Fig. 1.(A) Probe and needle position to perform LTN block at the scalene level. (B) BP at the C6 level. The LTN is also visualized (asterisk). ASM: anterior scalene muscle, MSM: middle scalene muscle, BP: brachial plexus, LTN: long thoracic nerve. 
|
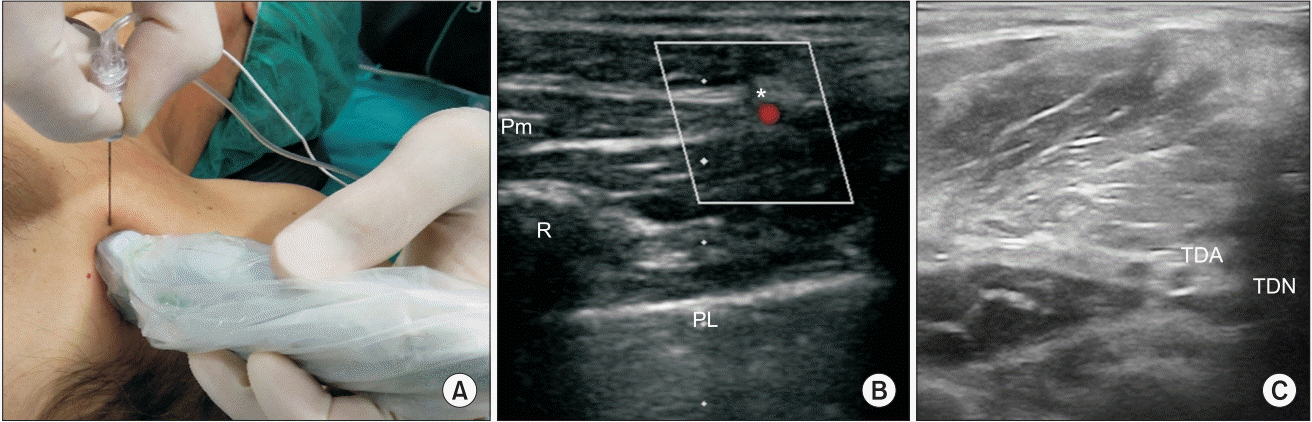 | Fig. 2.(A) Probe and needle position to perform LTN block at the axillary level. (B) Pm: pectoralis minor, R: rib, PL: pleural line, asterisk: Lateral thoracic artery and the LTN at the level of the anterior axillary line. (C) Thoracodorsal artery and the thoracodorsal nerve (TDN) are visualized by moving the probe posteriorly from its position in Panel A. LTN: long thoracic nerve, TDA: thoracodorsal artery. 
|
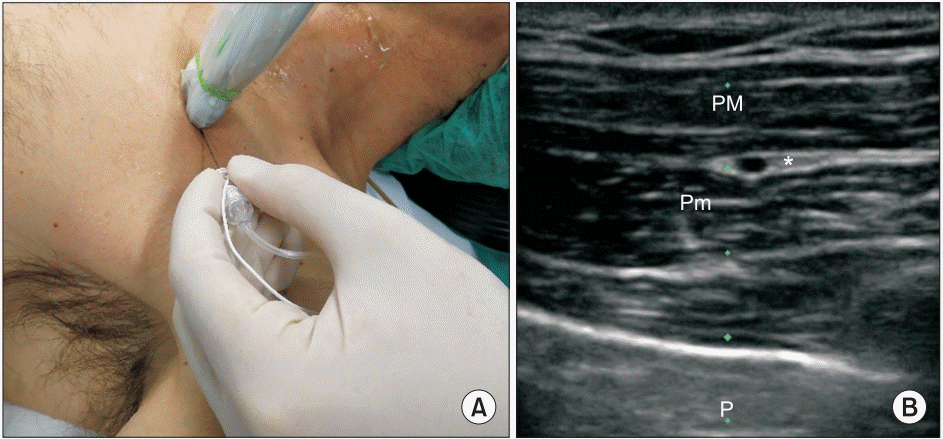 | Fig. 3.(A) Probe and needle position to perform LPN and MPN block (PECS I). (B) PM: pectoralis major, Pm: pectoralis minor, P: pleural line, asterisk: target, LPN: lateral pectoral nerve, MPN: medial pectoral nerve. 
|
An adequate level of anesthesia was ensured on assessment after 30 min by testing for pain sensation to pinprick (
Fig. 4), followed by the commencement of the surgical procedure. During surgery, a score of 3–4 was achieved on the Observer’s Assessment of Alertness/Sedation scale with a bolus dose of propofol 40 mg followed by an infusion of 2 mg/kg/h. No intraoperative opioids were required, and the patient remained comfortable throughout the entire duration of surgery that lasted for about 100 min. Administration of 1 g acetaminophen every 8 h was performed for postoperative pain relief; no additional analgesics were required intraoperatively and for two postoperative days before discharge. Postoperative pain was assessed every 6 hours using the numerical rating scale (NRS). The patient reported a pain score of less than 3 on the NRS at each pain assessment. He was discharged on the second postoperative day without complications.
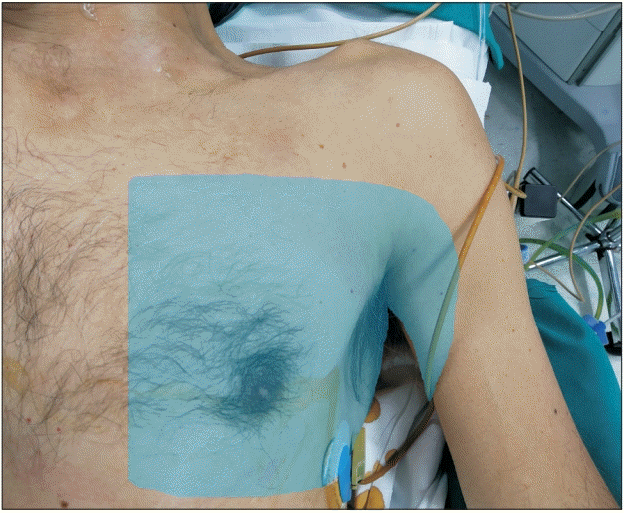 | Fig. 4.Sensory distribution of the block. 
|
Go to :

Discussion
Although innervation of the breast is complex, it consists of two major components - the intercostal nerves (T2–T6) and branches of the brachial plexus including the MPN, LPN, LTN and the TDN [
5].
Over the past few years, several techniques have been proposed to provide analgesia for breast surgery including epidural, paravertebral, and PECS block; however the ESP block has recently emerged as a novel technique with several advantages. The ESP block is easy to perform, the site of needle insertion is lateral to the neuraxis, and it provides somatic and visceral analgesia by spread in paravertebral space.
The ESP block was initially developed to treat chronic thoracic pain. It has been used for a wide range of indications from carotid endarterectomy to hip surgery [
6,
7]. The block is performed with the patient in the sitting position. After identification of the chosen vertebral level, a high-frequency linear ultrasound probe is used to identify the transverse process (about three centimeters lateral to the spinous process). Three layers of muscle are identifiable at the thoracic level, including the trapezius, the rhomboid major, and the erector spinae. The needle is inserted in a cephalad-to-caudad or caudad-to-cephalad direction until the tip lies in the interfascial plane between the rhomboid major and the erector spinae muscles. The needle position is confirmed by a linear spread of fluid between the muscles during injection.
The ESP block aims to anesthetize both the ventral and the dorsal rami of the spinal nerves by spread in the ESP and in the paravertebral space through the costotranserve foramen [
1]. Forero et al. [
8] have demonstrated that ESP block performed at the T2–T3 level blocks the brachial plexus. However, it remains unclear which part of the brachial plexus is blocked and whether this is a consistent effect with ESP block. Besides, a block carried out at the T2 level may be ineffective in blocking the lower dermatomes adequately. Recently, Kimachi et al. [
3] using a novel anesthetic technique, performed a radical mastectomy under ESP block and demonstrated its efficacy in blocking all the nerves involved. However, Ueshima and Otake [
9] reported that ESP block alone was not sufficient to achieve adequate anesthesia of the anterior branches of T2–T6.
The combination of a PECS II block with ESP block [
4] has been reported to be an effective technique to provide anesthesia for radical mastectomy and axillary clearance. PECS II is an interfascial block proposed by Blanco et al. [
10] and is a modification of the PECS I block. The PECS I block targets the LPN and the MPN at the interfascial plane between the pectoralis major and minor muscles and is performed by a single injection of local anesthetic. The PECS II block is performed by a double injection technique (10 and 20 ml of local anesthetic) and aims to block the pectoral nerves, the thoracodorsal nerve, 3rd to 6th intercostals nerves and the LTN. The ESP block aims to block intercostals nerves with spread inside paravertebral space. However, there are two important considerations. First, there is an overlapping effect of the PECS II and ESP blocks on the intercostal roots; second, the total volume of local anesthetic needed to perform a combined block is extremely high (50 ml) and could trigger systemic toxicity from local anesthetic dose. We developed the SBP block combined with ESP block (or paravertebral block) to provide complete anesthesia to the breast and the axillary region using a lower volume of local anesthetic. This technique involves blocking the LTN, TDN, MPN, and LPN. The LTN originates from the ventral rami of C5, C6, and C7 and descends between the anterior and middle scalene muscles; subsequently, it courses on the chest wall along the mid-axillary line and passes superficial to the serratus anterior muscle [
11].
We propose two approaches to LTN block. In our experience, the first option is to perform the block at the C6–C7 level where the LTN is identifiable in about one-third of patients [
11]. The LTN lies within the middle scalene muscle at an average distance of 0.7 cm from the brachial plexus. It has a hyperechoic appearance on ultrasound with a small hypoechoic area within (
Fig. 1). It is important to note that in some patients two structures are visible in the same plane; the more superficial structure is the dorsal scapular nerve while the deeper structure is the LTN.
If the LTN cannot be visualized in the supraclavicular region, a second approach proposed by Chang et al. [
12] may be used to identify the nerve in the infraclavicular or axillary region. After abduction of the ipsilateral arm, the linear transducer is placed on the midaxillary line in a horizontal plane. A useful landmark is the lateral thoracic artery identified by Doppler interrogation. The LTN is seen as a hypoechoic ovoid structure near the artery, usually lateral to the pectoralis minor (
Figs. 2A and
2B). It should be followed to perform the block as cranially as possible. The TDN originates mainly from the rami of C7 and C8 with some nerve fibers from C6. It passes below the axillary vein, descends into the axilla, and appears adjacent to the thoracodorsal artery, which is a branch of the axillary artery [
12,
13]. The thoracodorsal artery may be used as a landmark (
Fig. 2C). The power Doppler mode may be used to identify the thoracodorsal artery; the nerve is seen adjacent to the artery as a hypoechoic ovoid structure in its short axis. It is important to remember that the thoracodorsal artery courses posterior to the lateral thoracic artery. After identification of the lateral thoracic artery (
Figs. 2A and
2B) it is possible to identify the thoracodorsal artery by moving the probe laterally towards the axillary region.
The MPN and the LPN are two major nerves that arise from the brachial plexus and innervate the pectoral muscles. MPN originates from C8 and T1 while LPN originates from C5, C6, and C7. LPN is the larger of the two nerves and lies between the pectoralis major and minor muscles. The MPN pierces the pectoral muscles to reach the lower third of the pectoralis major [
11]. The MPN and the LPN are blocked using the approach proposed by Blanco et al. for the PECS I block [
10]. In this approach, the local anesthetic is injected in the plane between the pectoralis major and minor muscles using the thoracoacromial artery as a landmark (
Fig. 3). The volume of 0.5% ropivacaine that we suggest to perform the blocks are as follows: 2–4 ml for the LTN, 2–4 ml for the TDN, and 10 ml as a single injection for the MPN and the LPN.
Despite these advantages, the combination of ESP and SBP block has several limitations. This combination is based on the assumption that the ESP block will provide complete anesthesia of the spinal roots; however, as reported previously by Ueshima and Otake [
9] this is not a consistent effect. Furthermore, to perform the SBP block, the four nerves arising from brachial plexus or their landmarks should be identifiable, which may not always be possible. In our experience, the SBP block is more difficult to perform in obese patients and in patients with a low muscle mass. Another limitation of this technique is the requirement for four injections which may lead to more patient discomfort compared to other techniques.
In conclusion, the SBP block is a novel technique that may provide anesthesia to the branches of the brachial plexus involved in innervation of the breast region. Further studies will be needed to verify its feasibility in breast surgery.
Go to :







 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download