Introduction
Materials and Methods
Results
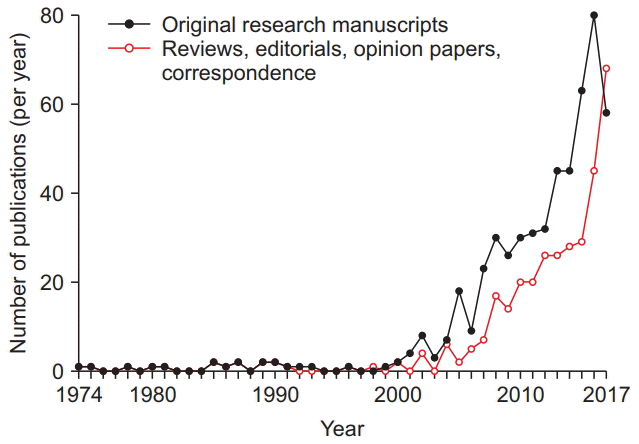 | Fig. 1.Research articles investigating the effects of anesthetic exposure on the developing animal brain and commentaries have increased significantly over the past 48 years. The number of animal studies (filled black circles), as well as review articles, editorial views, commentaries, opinion papers, and correspondence on this topic (unfilled red circles) that have been published annually, as identified in a literature search using PubMed and Scopus databases, are shown spanning from 1974 until 2017. |
Table 1.
| Study population (calendar years of birth or treatment) | Study group (s)/Control group | Anesthetic drugs | Duration of exposure | Number of subjects/controls | Age during exposure | Age during assessment | Neurological assessment tools | Neurological abnormalities observed in study group | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Prospective treatment cohort (1997) | Prolonged sedation and/or analgesia for intensive care vs. no exposure to sedation or analgesia | No data | Not specified | 97 exposed vs. 1,248 controls | Neonatal period in premature infants born 22-32 weeks PCA | 5 years | MPC | No difference in disability after adjustment for severity of illness; study lacked power to detect < 10% difference | Rozé et al. [11] |
| Retrospective treatment cohort (1987, 1991, 1993, 1995) | General anesthesia for urological procedures before 24 months vs. after 24 months of age | No data | Not specified | 178 under, 65 > 24 months | 0-6 years | > 4 years | CBCL/4–18 | Incidence of behavioral disturbances higher in children operated on < 24 months of age, compared with > 24 months, but not statistically significant | Kalkman et al. [12] |
| Birth cohort (1976-1982) | General anesthesia before age 4 vs. no anesthetic exposure | Predominantly halothane, nitrous oxide, ketamine, and/or thiopental; doses not quantified, stratified by number of exposures for mostly ENT and general surgery | 125 ± 227 min median (IQR) 75 (45–120) | 593 exposed vs. 4,764 controls | 0-4 years, most 2 years | ≤ 19 years | Review of medical and educational records several years after exposure | Doubling of the risk of learning disabilities following 2 or more surgical procedures with anesthesia for more than a combined 2 hours, compared with no or one exposure | Wilder et al. [13] |
| Birth cohort (1976-1982) | General anesthesia vs. regional anesthesia for cesarean delivery vs. spontaneous vaginal birth | Predominantly sodium thiopental, nitrous oxide, and halothane; doses not specified | Median 14 min (IQR 8-23) | 5,320 | At birth | ≤ 19 years | Review of school/educational records | Similar incidence of learning disabilities among children delivered vaginally without anesthesia and via cesarean section with general anesthesia, but lower following cesarean section with regional anesthesia | Sprung et al. [14] |
| Birth cohort (1999-2001) | ICD-9 code for Inguinal hernia repair vs. absence of code | No data | Not specified | 383 exposed vs. 5,050 controls | 0–3 years | ≤ 4 years | ICD-9 diagnostic codes indicating neurological abnormalities | Increased incidence of developmental or behavioral disorder, mental retardation, autism, and language or speech problems | DiMaggio et al. [15] |
| Review of data from Young Netherlands Twin Register (1986-1995) | No data | No data | Not specified | 154 exposed vs. 648 individual controls | 0–3 years | 12 years | Educational achievement (Cito) and Connor Teacher Rating Scale | Educational scores lower in concordant twin pairs exposed to anesthesia compared with unexposed pairs. No difference in educational achievement comparing both twins in pairs discordant for exposure | Bartels et al. [16] |
| Case-control study (1999-2005) | ICD-9 procedural codes for surgery vs. absence of code | No data | Not specified | 304 exposed vs. 10,146 controls | 0–3 years | ≤ 4 years | ICD-9 diagnostic codes indicating neurological abnormalities | Increased incidence of developmental or behavioral disorders following ≥ 2 exposures under 3 years of age | DiMaggio et al. [17] |
| Birth cohort (1976-1982) | Vaginal delivery with vs. without regional anesthesia | No data | Not specified | 1,495 exposed vs. 3,189 controls | At birth | ≤ 19 years | Review of school/educational records | No association of regional anesthesia with learning abnormalities | Flick et al. [18] |
| Birth cohort, matched design (1976-1982) | General anesthesia before age 2 vs. no anesthetic exposure | Halothane, N2O, not quantified | 133 ± 239 min (median: 75 min | 350 exposed vs. 700 controls | 0–2 years | ≤ 19 years | Review of school/educational records | Exposure to multiple, but not single anesthetics increased the risk of developing a learning disability | Flick et al. [19] |
| Prospective treatment cohort (2003-2006) | Sedation, anesthesia, analgesia for congenital heart surgery vs. population norms | Doses for ketamine, benzodiazepines, chloral hydrate, opioids, inhalation agents quantified | Not specified | 95 | ≤ 6 weeks | 18–24 months | BSID-II and BSID-III | No evidence for an association between sedation/analgesia variables and mental, motor, or vocabulary delay | Garcia Guerra et al. [20] |
| Nationwide birth cohort (1986-1990) | Inguinal hernia repair vs. randomly selected 5% population sample | No data | Not specified | 2,689 exposed vs. 14,575 controls | Infancy | 15–16 years | Academic performance during 9th grade exam and teacher rating | No significant differences in test scores; increased non-attainment was attenuated when adjusting for congenital malformations | Hansen et al. [21] |
| Birth cohort (1989-1992) | Anesthetic exposure vs. no exposure | No data | Not specified | 321 exposed vs. 2,287 controls | 0–3 years | ≤ 10 years | CELF, CPM | Increased risk for language and cognitive disability even after single exposure | Ing et al. [22] |
| Cohort study | Inguinal hernia repair, orchidopexy, pyloro-myotomy, and/or circumcision vs. population norms | No data | Range 1-12 hours | 58 | Infancy | 7–10 years | Academic performance (Iowa Tests of Basic Skills and Educational Development) | Patients without potential risk factors for preexisting CNS abnormalities did not score lower than the population norm, but scored below the 5th percentile more than the Iowa population | Block et al. [23] |
| Longitudinal, observational cohort (2001-2004) | Infant surgeries, including inguinal hernia repair, PDA ligation, laparotomy vs. non-surgical patients in same cohort | No data | Not specified | 30 exposed vs. 178 controls | Neonatal period in premature infants (< 33 weeks) | 2 years | BSID-II | No difference in PDI scores, but lower MDI scores, which were non-significant after adjustment for confounders | Filan et al. [24] |
| Retrospective observational cohort study | General anesthesia for inguinal hernia repair, circumcision, cystoscopy, pyloromyotomy vs. no exposure | Sevoflurane | 30–120 min (20% repeat exposures) | 100 exposed vs. 106 controls | < 1 year (4.4 ± 3.2 mo) | 12 years | Formal diagnosis of learning disability | No difference in PSLE aggregate scores, but odds for diagnosis oflearning disability 4.5 times greater than for unexposed peers | Bong et al. [25] |
| Nationwide birth cohort (1986-1990) | Pyloromyotomy vs. randomly selected 5% population sample | No data | Not specified | 779 exposed vs. 14,665 controls | 0–3 months | 15–16 years | Academic performance during 9th grade exam and teacher rating | No significant differences in test scores; increased non-attainment was attenuated when adjusting for VLBW infants | Hansen et al. [26] |
| Prospective treatment cohort | Strabismus surgery, cognitive performance before vs. after surgery | sevoflurane, fentanyl (not further quantified) | 67.3 ± 9.8 min | 72 | 4–7 years | 1 day before, 1 month, and 6 months after procedure | WPPSI-III | No decrease in postoperative cognitive ability when compared with preoperative performance | Fan et al. [27] |
| Retrospective treatment cohort | Neonates undergoing complex congenital heart surgery vs. population norms | Doses for anesthetics and opioids quantified | 4.4 MAC-hours | 59 | Infancy | 12 months | BSID-III | Lower cognitive scores were associated with higher volatile anesthetic exposure | Andropoulos et al. [28] |
| Prospective treatment cohort (2003-2006) | Sedation/Anesthesia/Analgesia for congenital heart surgery vs. population norms | Doses for ketamine, benzodiazepines, chloral hydrate, opioids, inhalation agents quantified | 84 hours mean chloral hydrate exposure | 91 | ≤ 6 weeks | 54 months | WISC-III, VMI-V, Adaptive Behavior Assessment System-II | Lower visual motor integration was associated with higher cumulative benzodiazepine dose and lower performance IQ with higher chloral hydrate dose | Garcia Guerra et al. [29] |
| Australian birth cohort (1989-1992) | Anesthetic exposure vs. no exposure | No data | Not specified | 112 exposed vs. 669 controls | 0–3 years | ≤ 10 years | CELF, CPM, ICD-9 code, academic achievement | Increased risk for deficit as measured by directly administered neuropsychological test or ICD-9 diagnosis, but not by academic achievement scores | Ing et al. [30] |
| Prospective test-retest study | Before vs. following last anesthetic exposure for laser treatment | Ketamine (8 mg/kgIM) | 5 min for 1–3 exposures | 49 exposed (20 once, 16 twice, and 13 three times) | 10–12 months | < 2 years | BSID-II | Decrease in mental development or psychomotor indices comparing baseline vs. following third exposure; no difference for 1–2 exposures; increase in S-100b plasma levels following all 3 exposures | Yan et al. [31] |
| Nationwide, retrospective matched-birth cohort study (2001-2005) | Anesthetic exposure vs. no exposure | No data | Not specified | 3,293 exposed vs. 13,172 unexposed | < 3 years | > 3 years | Diagnosis of Attention Deficit Hyperactivity Disorder (ICD- 9-CM code 314.01) | No association with the diagnosis of ADHD | Ko et al. [32] |
| Nationwide, retrospective birth cohort study (2001-2007) | Anesthetic exposure vs. no exposure | No data | Not specified | 5,197 exposed vs. 20,788 unexposed | < 2 years | 3–9 years | Diagnosis of autism (ICD-9-CM 299.00) | No association with the diagnosis of autistic disorder | Ko et al. [33] |
| Matched cohort study | Anesthetic exposure for mostly urological surgeries vs. no exposure | Propofol, N2O, volatile anesthetics | Not specified | 28 vs. 28 | < 1 year most around 10 months | 6–11 years | Recognition memory Tasks, WASl, CBCL | Recollection memory significantly lower in both color and spatial tasks, no difference in familiarity, IQ or behavioral indices | Stratmann et al. [34] |
| Cohort study matched for gender and age | Anesthetic exposure for predominantly urological surgery vs. no exposure | No data | > 1 hour 65–317 min 160 ± 75 median 142 min | 15 vs. 15 | < 2 year | 10–17 years | fMRI activity (‘go/no-go’ attention task) | No differences in performance accuracy and response time; activation differences detected in cerebellum, cingulate gyrus, and paracentral lobule. | Taghon et al. [35] |
| Nationwide, retrospective birth cohort study (2004-2007) | Cesarean section with general anesthesia (GA) vs. with regional anesthesia (RA) vs. vaginal birth | No data | Not specified | 12,384 GA vs. 161,992 RA vs. 362,297 vaginal | At birth | 2–6 years | Diagnosis of autism (ICD-9-CM code 299.0) | Incidence of autism higher in neonates delivered by C-section with GA than in neonates delivered vaginally or with regional anesthesia | Chien et al. [36] |
| Cohort study matched for gender, age, handedness, and socioeconomic status | Anesthetic exposure for predominantly ENT surgery vs. no exposure | Halothane, N2O, sevoflurane | 37 ± 37 min (range 5–170 min) | 53 exposed vs. 53 controls | < 4 years | 5–18 years | WISC/WAIS, OWLS, MRI | Lower scores in listening comprehension and performance IQ, which was associated with lower gray matter density in the occipital cortex and cerebellum | Backeljauw et al. [37] |
| Randomized controlled trial | Inguinal hernia repair with general anesthesia (GA) vs. with regional anesthesia (RA) | Sevoflurane (2.6%/ hour) vs. caudal or spinal | 54 min (41-70) | 294 GA vs. 238 RA | < 1 year 2.3 ± 1 months | 2 years | BSID-III | No differences between groups | Davidson et al. [38] |
| Sibling-matched cohort study | Inguinal hernia repair with general anesthesia (GA) vs. unexposed sibling | No data | 84 ± 33 min median 80 min range 20–240 min | 105 GA (10% female) vs. 105 unexposed (44% female) | < 3 years 17 ± 11 months | 8–15 years | WASI, NEPSYII, WISC-IV, CBCL | No statistically significant difference in full-scale IQ score between siblings with and without a single anesthesia exposure before age 3 years | Sun et al. [39] |
| Nationwide birth cohort (1986-1990) | Cleft lip and or palate repair vs. 5% sample of birth cohort | No data | Not specified | Cleft lip (171), cleft lip and palate (222), or cleft palate (195)/controls (14,677) | Median age 2.8 months for CL and CLP, 22.1 months for CP | 15–16 years | Danish standardized 9 th-grade exam | Lower scores only in children with cleft palate repair, after adjustment for sex, birth weight, parental age, and parental level of education | Clausen et al. [40] |
| Cohort study, matched for gender, gestational age at and year of birth, mothers age, and rurality of residence | General anesthesia before age 4 vs. no anesthetic exposure, excluding children with known behavioral, learning, and developmental disability | No data | Not specified | 28,366 exposed/55,910 controls (36% female) | 0–6 years | 5–6 years | EDI | Reduced physical health and wellbeing, social knowledge and competence, and emotional health and maturity scores in older age group (> 2 years during exposure) | O’Leary et al. [41] |
| Cohort study, matched for gender, gestational age at birth, socioeconomic status | General anesthesia in the neonatal period for correction of gastrointestinal malformations | No data | 145 min (95% CI 115–175 min) | 40 exposed (63% male)/40 controls (63% male) | < 28 days | 2 years | BSID-II | No difference in PDI, but significantly lower verbal scale of MDI | Doberschuetz et al. [42] |
| Matched cohort study, matched for gestational age | General anesthesia before age 4 vs. no anesthetic exposure, excluding children with developmental disabilities (DD) diagnosed prior to 5 years | No data | Not specified | 3,850 single, 620 multiple, 13,586 no exposures | 0–4 years | 5 years | EDI | Deficits in communication/ general knowledge and language/cognition parts of EDI following exposure between 2-4, but not 0-2 years | Graham et al. [43] |
| Nationwide birth cohort (1973-1993) | Single vs. multiple vs. no anesthetic exposure before age 4 | No data | Not specified | 33,514 single, 3,640 multiple, 159,619 matched | 0–4 years | 16/18 years | School grades (age 16) and IQ test during military conscription test (age 18) | Small decrease in school grades by 0.12–0.7% or IQ by 0.15–1.8%, strongest effect for exposures between 37–48 or 25–36 months of age, respectively. No differences between number of exposures | Glatz et al. [44] |
| Birth cohort (1994-2007), propensity-matched | Single vs. multiple vs. no anesthetic exposure before age 3 | Sevoflurane, N2O, isoflurane, not quantified | None vs. 61 ± 51 vs. 295 ± 354 min (median: 45 vs. 187 min | exposures 380 single and 206 multiply exposed vs. 411 controls | 0–3 years | 8–12 or 15–20 years | WASI, CTOPP, Fine Motor Composite, | Single or multiple exposures did not diminish subsequent IQ, processing speed and fine motor skills were decreased in multiply, but not singly exposed | Warner et al. [45] |
BSID-II or BSID-III: Bayley Scales of Infant Development, 2nd or 3rd Edition, CBCL: Achenbach Child Behavior Checklist Parental Assessment, CELF: Clinical Evaluation of Language Fundamentals, CP: Cerebral Palsy, CPM: Raven’s Colored Progressive Matrices, CTOPP: Comprehensive Test of Phonological Processing, EDI: Early Development instrument, ELBW: Extremely Low Birth Weight (< 1,000 g), FSIQ: Full Scale Intelligence Quotient, ICD: International Statistical Classification of Diseases, IM: intramuscular, IQ: Intelligence Quotient, MDI: Bayley Mental Development Index, MPC: Mental Composite Score of the Kaufman Assessment Battery for Children, NEPSY-II: Developmental Neuropsychological Assessment, 2nd Edition, PCA: Postconceptual Age, PDA: Patent Ductus Arteriosus, PDI: Bayley Psychomotor Developmental Index, VMI: Developmental Test of Visual Motor Integration, VLBW: Very Low Birth Weight (≤ 1,500 g), WAIS: Wechsler Adult Intelligence Scales, WASI: Wechsler Abbreviated Scales of Intelligence, WISC-III: Wechsler Intelligence Scale for Children, 3rd Edition, WISC-IV: Wechsler Intelligence Scale for Children, 4th Edition, WPPSI: Wechsler Preschool and Primary Scales of Intelligence.
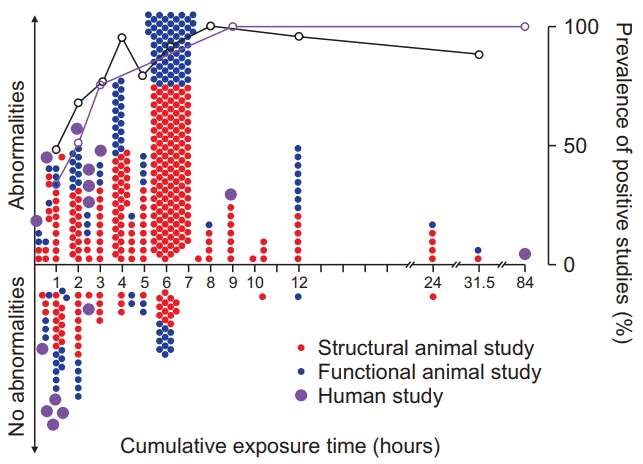 | Fig. 2.The majority of in vivo animal studies on the effects of anesthetic exposure on brain structure and function examine exposure times greater than occurring during routine clinical care. However, the prevalence of both animal and human studies returning positive findings, thereby suggesting potential injury, increases similarly with exposure time. Animal studies reporting abnormal findings are depicted above the horizontal line and negative findings are shown below the horizontal line, for both brain structural (red filled circles) or cognitive outcomes (blue filled circles), as a function of exposure time. Purple filled circles represent clinical studies into long-term cognitive outcomes in humans demonstrating abnormalities (above horizontal line) or no abnormalities (below horizontal line). Exposure times denote all reported anesthetic durations, either single or cumulative, or estimated exposure times based on injection schema, as described in the methods; accordingly, only studies reporting exposure times or injection schemata were included in the analysis. For the purpose of analysis, results are reported separately for each condition or outcome studied, and therefore single studies may be represented by multiple data points. Graphs express the ratio of positive to negative studies in animals (black) and humans (purple); open circles represent percentage of positive studies for respective exposure epochs between data points. Positive studies outnumber negative studies for all exposure times in both animals and humans, except for anesthetic exposures of 1 hour or less. |
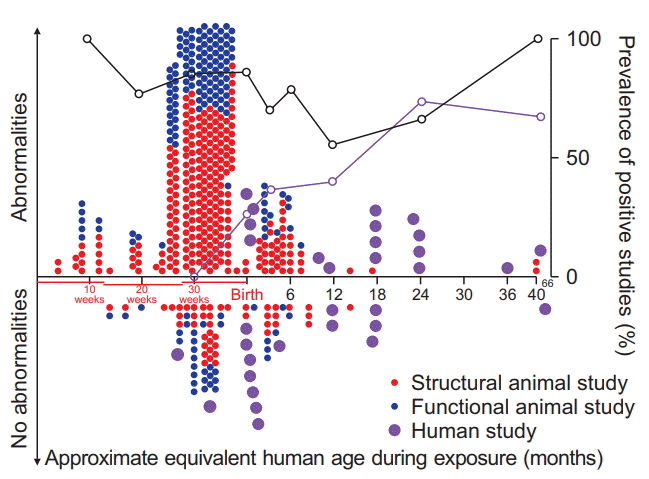 | Fig. 3.The majority of in vivo animal studies on the effects of anesthetic exposure on brain structure and function focus on brain developmental stages equivalent to premature neonates. For animals, prevalence of positive studies outnumber negative studies for all age brackets, suggesting that no age can be clearly identified at which anesthetics do not cause abnormalities. Animal studies reporting abnormal findings are depicted above the horizontal line and negative findings are shown below the horizontal line, for both brain structural (red filled circles) and cognitive outcomes (blue filled circles), as a function of maturational age of the animals’ brain relative to the human brain during exposure. Purple filled circles represent clinical studies into long-term cognitive outcomes in humans. The animals’ age during exposure was converted to the corresponding maturational stage of the human brain based on a computational neurodevelopmental model [8-10] (www.translatingtime.net, translating ‘neurogenesis’ for ‘whole brain,’ last accessed 4/1/2018). For the purpose of analysis, reports with separate anesthetic protocols consisting of different anesthetic regimens or multiple exposure times of the same anesthetic were classified as separate studies. For repeated exposures at different ages, the equivalent mean age of the human brain is reported. Graphs express the ratio of positive to negative studies in animals (black) or humans (purple); open circles represent the respective percentage of positive studies for age epochs ranging from specific data points to the next lower data point on the graph. |
Discussion
Does anesthetic exposure harm the human brain?
What are the structural abnormalities observed in animals, and has an exposure threshold been identified below which no injuries occur?
Does animal research demonstrate all general anesthetics to be similarly toxic or are some drugs safer to use than others?
What might explain the specific vulnerability of the developing brain to the anesthetics’ deleterious effects?
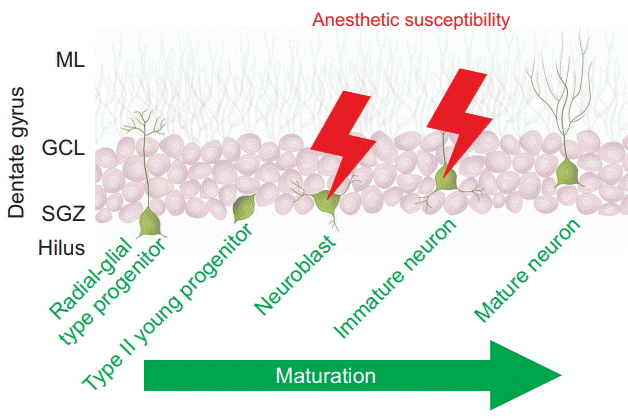 | Fig. 4.Neurons during intermediate stages of their development are specific targets of anesthesia-induced apoptosis During the continuum of neuronal maturation in the dentate gyrus, a brain region with life-long neurogenesis in rodents, isoflurane triggers apoptotic cell death in neuroblasts and immature neurons residing in the neurogenic niche, the subgranular zone (SGZ), but not in more immature radial-glial type and type II progenitors, or in mature neurons, which reside in the granule cell layer (GCL) and extend dendrites into the molecular layer (ML). Data are derived from Hofacer et al. [65]. The illustration depicts five representative stages of neuronal maturation throughout the life cycle from a pluripotent radial-glial type progenitor cell on the left to a postmitotic, mature neuron on the right. Cells at these various stages coexist in the postnatal dentate gyrus. |
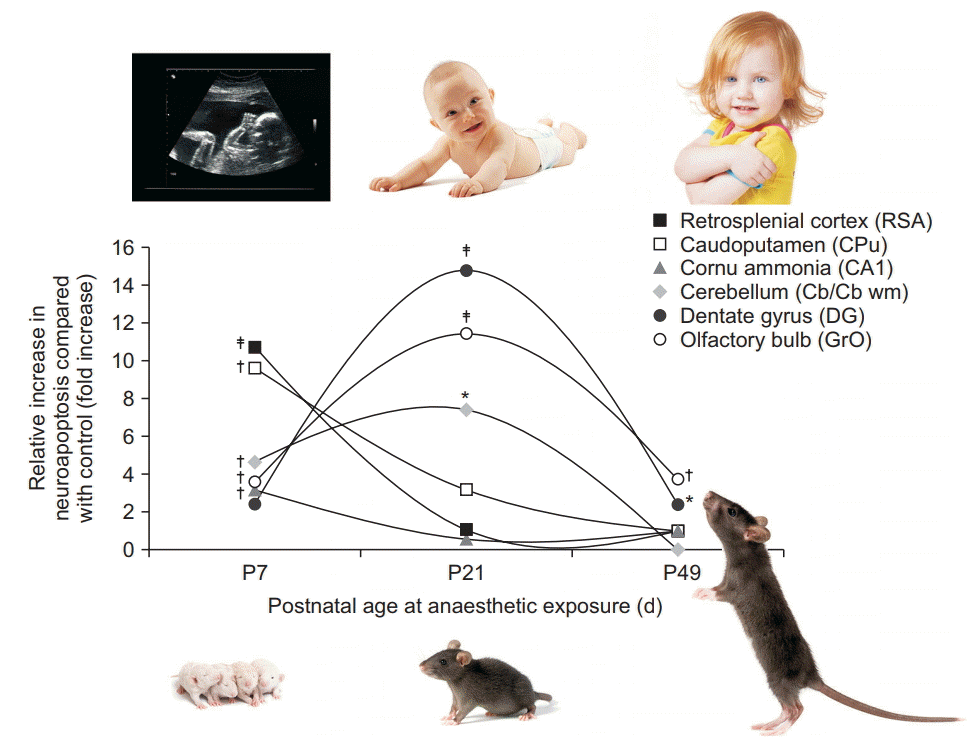 | Fig. 5.Brain regional vulnerability to anesthesia-induced neuroapoptosis changes with age during exposure. Graphs indicate the relative increase in neuronal cell death observed at three different ages following isoflurane exposure, relative to physiological apoptotic cell death observed in littermate controls. Apoptotic neuronal density was quantified in superficial layers II/III of the retrosplenial agranular cortex (RSA), caudoputamen (CPu), the pyramidal layer of cornu ammonis 1 (CA1), cerebellum (Cb at P7, CB wm at P21), the subgranular zone and granule cell layer of dentate gyrus (DG), and the granule layer of the olfactory bulb (GrO) after a 6 h exposure to 1.5% isoflurane in newborn (P7), juvenile (P21), and adult mice (P49), compared with fasted, unanesthetized littermates [66]. Maximum vulnerability was observed in the neocortex, caudoputamen, and cornu ammonis 1 at P7, whereas the number of vulnerable neurons peaked at P21 for the cerebellum, dentate gyrus, and granule layer of the olfactory bulb. The murine brain’s maturational stage at P7 approximates the premature human brain, at P21 the infant brain, and at P49 the older child’s or adolescent brain [10]. *P < 0.05, †P < 0.01, ‡P < 0.001 compared with control. Adapted from Deng et al. [66]. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download