Abstract
Delirium can be defined as an ‘acute brain dysfunction.’ Compared to dementia, which is a disease that deteriorates the brain function chronically, delirium shows very similar symptoms but is mostly ameliorated when the causative factors are normalized. Due to the heterogeneity in etiologies and symptoms, people including health care workers often mistake delirium for dementia or other psychiatric disorders. Delirium has attracted global interest increasingly and a vast amount of research on its management has been conducted. Experts in the field have constantly suggested that systematic intervention should be implemented through a team-based multicomponent approach aimed to reduce the incidence and duration of delirium. Surgery involves many health care workers with different expertise who are not familiar with delirium. For a team-based approach on the management of delirium, it is vital that all medical personnel concerned have a common understanding of delirium and keep in constant communication. Postoperative delirium is a common complication and exerts an enormous burden on patients, their families, hospitals, and public resources. To alleviate this burden, this article aimed to review general features and the latest evidence-based knowledge of delirium with a focus on postoperative delirium.
Delirium has attracted global interest increasingly and a vast amount of research on its management has been conducted. Experts in the field have constantly suggested that systematic intervention should be implemented through a team-based multicomponent approach aimed to reduce the incidence and duration of delirium [1,2]. Surgery involves many health care workers with different expertise who are not familiar with delirium. For a team-based approach on the management of delirium, it is vital that all medical personnel concerned have a common understanding of delirium and keep in constant communication. This article aimed to review general features and the latest evidence-based knowledge of delirium with a focus on postoperative delirium.
Delirium can be defined as an ‘acute brain dysfunction.’ Compared to dementia, which is a disease that deteriorates the brain function chronically, delirium shows very similar symptoms but is mostly ameliorated when the causative factors are normalized. While the reversibility of delirium is expressed as ‘acute,’ the words ‘brain dysfunction’ connote the diversity of symptoms. Any possible symptom related to the brain such as disorientation, perceptual disturbances, emotional dysregulation, or sleep disturbances could appear in delirium.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) defines the key feature of delirium as a disturbance in attention and awareness [3]. As described among the full diagnostic criteria by DSM-5 listed in Table 1, another important factor of delirium is an acute and fluctuating course of mental state. Postoperative delirium usually happens in the recovery room and appears up to 5 days after surgery [4,5]. One study discovered that many patients who showed postoperative delirium on the peripheral ward had delirium in the recovery room [5]. Emergence delirium refers to the delirium in the immediate post-anesthesia period [6,7].
Clinical subtyping of delirium according to motor activity is widely used [8,9]. Hypoactive delirium is characterized by decreased activity, reduced alertness, withdrawal, unawareness, and decreased speech while hyperactive delirium shows agitation, wandering, irritability, and hallucination [10]. Mixed delirium shows both hyperactive and hypoactive features in short time frames.
Some evidence suggested that certain risk factors such as preexisting cognitive impairment, older age, frailty, and severity of physical illness are more associated with hypoactive delirium [11,12]. More systematic research is required to elucidate the relationship between predisposing factors and motor profile. Due to the absence of overt distress or disturbance in hypoactive delirium, hypoactive delirium is more likely to be overlooked than hyperactive delirium. Worse prognosis has been reported in hypoactive delirium possibly due to the difficulty of detection and subsequent delayed treatment [9].
To use a reference standard such as the DSM-5, users must undergo extensive training. To detect and evaluate delirium easily and quickly, many assessment tools have been developed. The Confusion Assessment Method (CAM) [13] is a screening tool that consists of 4 features: (a) an acute onset and fluctuating course of mental state, (b) inattention, (c) disorganized thinking, and (d) altered level of consciousness. Delirium is diagnosed when features (a) and (b) are satisfied essentially and (c) or (d) selectively. The CAM for the intensive care unit (ICU; CAMICU) is a two-minute version of the CAM to be administered easily in the ICU with an accuracy of over 93% [14,15]. Skilled personnel with proper education on the tool should be able to apply the CAM with high sensitivity [16].
The Richmond Agitation-Sedation Scale (RASS) is a tool to assess the level of sedation/agitation [17]. As the DSM-5 guidance notes stated that a severely reduced level of arousal (of acute onset) above the level of coma should be considered as having ‘severe inattention’ and hence as having delirium [3,18], the RASS can be useful in diagnosing delirium.
The Memorial Delirium Assessment Scale (MDAS) [19] and the Delirium Rating Scale-Revised-98 (DRS-R98) [20,21] are also useful in evaluating the presence and severity of delirium but are more time-consuming than the CAM. The MDAS focuses on the assessment of disturbances in consciousness and cognitive function (10 items with a total score ranging from 0 to 30). The DRS-R98 includes a relatively wider scope of symptoms, containing 3 diagnostic items and 13 severity items (total score ranging from 0 to 46, with higher score indicating more severe delirium). The original validation study suggested that 15 points or more on the severity scale would indicate dementia or other psychiatric disorders [20].
For the routine implementation of assessment tools for delirium, it is essential to train personnel on the basic features of delirium as well as the features and characteristics of the scales. This is not merely because tools such as the CAM require education, but also because the personnel should have a common understanding of delirium and keep in constant communication on the results of the tools [22,23].
Apart from clinical diagnostic tools, previous studies have shown evidence of biomarkers for the detection and monitoring of postoperative delirium in both ICU [24,25] and non-ICU settings [26,27]. However, further research is required to apply them in practice. Electroencephalography (EEG) can also be used as a relatively objective diagnostic tool for delirium. It is widely known that patients show a slowing of background activity as detected by EEG during delirium [28]. The routine use of EEG monitoring for delirium screening would be time-consuming and inefficient because of issues with the interpretation of the results. A recent EEG study using 2 electrodes and automatic processing showed a large difference between delirium and non-delirium in a homogenous population of non-sedated patients [29]. With specific protocols and proper automatic processing, EEG may provide consistent and objective diagnosis of delirium in the future.
The pathophysiology of delirium is poorly understood. In spite of many studies to find a common mechanism that explains all aspects of delirium, there may not be a single mechanism that could explain the whole syndrome with its heterogeneous etiologies and presentations. There are 2 leading hypotheses that has helped us to understand the complex nature of delirium [30]. The first emphasizes the role of inflammation, particularly the action of cytokines on the blood-brain barrier and the impact of chronic stress on cytokine and cortisol levels. The second highlights the neurochemical imbalances that affect neurotransmission. Neurochemical changes are found in the acetylcholine, dopamine, glutamate, gamma-aminobutyric acid, and serotonin systems. A recent neuroimaging study investigated the strength of resting-state functional connectivity between regions producing or utilizing acetylcholine and dopamine during and after an episode of delirium using functional magnetic resonance imaging (fMRI) [31]. When compared to the group without delirium, patients with delirium showed disruption in reciprocity of the dorsolateral prefrontal cortex with the posterior cingulate cortex and reversible reduction of functional connectivity of the subcortical regions [31]. Another fMRI study focused on the sleepwake disturbance in delirium and showed a dysregulation of the default mode network and mental coordination processing areas by the suprachiasmatic nucleus of the hypothalamus [32].
It is widely accepted that delirium occurs by the cumulative interactions between predisposing and precipitating factors [2]. Predisposing factors are generally considered potent predictors of delirium. The smaller the vulnerability a patient has at baseline, the more resistance to delirium he/she shows even under stressful conditions. On the contrary, with high vulnerability, a patient may easily develop delirium under little insult [30]. For example, considering that old age is a strong predictor of delirium, a patient aged more than 65 would experience delirium when exposed to just 1 or 2 precipitants [33]. With the same precipitating factors, younger patients may not have delirium or may stay in the subthreshold state. In younger patients, compared to elderly patients, delirium would develop under much more complex and varied interactions among the causative factors. The European Society of Anesthesiology presented the evidence-based and consensus statements for preoperative, intraoperative, and postoperative risk factors for postoperative delirium as listed in Table 2 [2]. Among the risk factors, pain is shown to be significantly correlated with anxiety and the dose of opioids administered in a recent study [34]. Since patients in the ICU commonly display anxiety [35] and prolonged administration of sedatives and analgesics to reduce anxiety may cause delirium [36], accurate evaluation and comprehensive management of both pain and anxiety is necessary to prevent delirium.
Additionally, emergency surgery [37,38] and postoperative complications raise the incidence and duration of postoperative delirium as well as the risk of long-term cognitive impairment [39]. To investigate these risk factors and to introduce strategies for risk reduction (e.g., fast track surgery) [23,40], a well-devised protocol needs to be developed. A report stated that hypothermia in the recovery room may be a risk factor for hypoactive emergence [41]. Also, the incidence of delirium after cardiac surgery was associated with preoperative fasting glucose concentrations [42,43].
As mentioned earlier in this article, clinical scoring tools such as the CAM and MDAS are based on the evaluation of the level of consciousness and changes in cognition. Sedative drugs are almost always used in patients who undergo surgery, but their interference with the assessment of delirium has not received much attention. One study showed that the apparent prevalence of delirium is dependent on how the level of consciousness is interpreted (persisting sedation despite the discontinuation of sedatives versus delirium) [44]. Another study reported that patients with rapidly reversible, sedation-related delirium did not differ from patients with no delirium in prognostic outcomes such as ventilator days, length of stay in the ICU, length of stay in the hospital, or hospital or 1-year mortality, whereas patients with persistent delirium showed worse outcomes [45]. It is not clear whether the effect of sedative drugs should be considered otherwise in diagnosing delirium since the diagnostic criteria according to the DSM-5 (criterion E) acknowledged multiple etiologies that affect attention and awareness. Setting aside diagnostic issues, the effect of sedative drugs should be closely monitored in delirium assessment.
Strong correlation between subjective emotional factors (e.g., pain and anxiety) and delirium is generally acknowledged [46]. Subjective emotional factors are mostly manifested on the basis of personality. Although various physical and medical risk factors for delirium have been identified, the effect of psychological factors has not been clearly studied so far. A recent study investigated the psychological risk factors for postoperative delirium to identify hidden subgroups of delirium. Phenotypic subgroups of delirium were assessed using Topological Data Analysis (TDA), and the results showed 4 predictive risk factors: the Mini-Mental State Examination score, neuroticism, conscientiousness, and regional anesthesia (Fig. 1) [47]. TDA is a clustering technique to discover the pattern or grouping in data while allowing overlaps among clusters [48]. Among the 5 personality factors proposed by Goldberg (extraversion, agreeableness, neuroticism, conscientiousness, and openness) [49], only 2 were shown to have an effect on the occurrence of delirium in the study by Shin et al. [47]; neuroticism and conscientiousness. Neuroticism is frequently used in affect studies and is generally considered to be related to negative affect [50]. People with high neuroticism tend to become miserable or to create unhappy situations. They are vulnerable to stress [51] and have poor emotional control [52]. Conscientiousness reflects self-control and people with high conscientiousness have a strong ability to control emotions [53]. The study using TDA mentioned above showed that patients with less self-control were more vulnerable to delirium [47]. It is an interesting and understandable result, but more research is necessary to elucidate the relationship between personality and delirium.
Delirium is often considered a transient brain dysfunction, and most patients achieve full remission. However, the results of previous studies strongly suggest that delirium is associated with long term cognitive [54] and non-cognitive morbidity [55], including a low quality of life [56].
Studies have shown that delirium in ICU patients was independently associated with worse prognosis [57,58]. In ICU patients, the prolonged duration of delirium was a strong indicator for a longer length of stay in the hospital and more days in the ICU [59]. Also, multiple studies have reported that the presence of delirium in ICU patients was associated with a reduced 6-month survival rate and cognitive decline on discharge [60,61]. Postoperative delirium, regardless of ICU admission, extends the total length of stay in the hospital [62–64]. Various studies have also reported the association between postoperative delirium and increased mortality [65–67].
Evidence has shown that postoperative delirium is associated with both short and long term cognitive impairment [54,68,69]. The trajectory of cognitive impairment following postoperative delirium is characterized by an initial decline and prolonged deterioration [54]. One study even suggested the association between postoperative delirium and dementia up to 5 years after delirium [70].
Thorough evaluation of patients before surgery for the presence of delirium and cognitive impairment is necessary because any cognitive impairment prior to hospital admission is an independent risk factor for long term cognitive impairment [71] and preoperative evaluation has shown prevalence rates of preexisting delirium up to 29.7% [72].
The progress and time course of delirium varies greatly due to its heterogeneous etiologies. According to the latest study, postoperative delirium developing as a secondary complication following surgery showed longer duration and length of hospitalization compared with postoperative delirium attributable to surgery and delirium in medical patients [73]. In the same study, as in Fig. 2 that shows the delirium recovery rate according to the time course, patients with delirium in medical wards showed lower delirium recovery rates at discharge than patients in surgical wards [73]. Along with the complex etiological nature of delirium, the findings above suggest that targeted screening and intervention are necessary.
The management of postoperative delirium is not so different from that of general delirium. Immediate treatment of both precipitating factors and symptoms is important in reducing the duration of postoperative delirium [65,74]. Current management approaches to delirium are mainly focused on the precipitants. However, if the precipitating factor is the surgery, which is inevitable, other approaches should also be considered. As for prevention, managing the predisposing factors of delirium is vital and would decrease the morbidity/mortality associated with delirium.
Non-pharmacological interventions are useful in both the prevention and management of delirium. They modify the patient’s surroundings to maximize the safety and calmness of the environment and to provide reassurance and decrease fear and agitation associated with delirium. Evidence-based approaches target 6 risk factors for multicomponent non-pharmacological intervention: cognition/orientation, early mobility, hearing, sleep-wake cycle preservation, vision, and hydration [75]. Orienting communication, minimization of immobilizing equipment, hearing aids, visual aids, environmental cues for normal sleep-wake cycles, and adequate nutrition are typical examples of non-pharmacological interventions. In addition to the care provided by the staff, family or close friends can be extremely important in the management of the patient’s symptoms. If regularly informed of the situation, family members can reassure the patient, provide reorientation, and reduce anxiety and agitation. Familiar items from home may be useful for selected patients. A recent meta-analysis study showed that multicomponent non-pharmacological delirium intervention is effective in reducing delirium incidence and preventing falls, with a trend toward decreasing the length of stay in hospitals and avoiding institutionalization [76].
The pharmacological management of delirium is not supported by strong evidence. It should be reserved for the management of behaviors associated with delirium that involves a safety risk for the patient and delirium due to drug or alcohol withdrawal. Two classes of medications are most frequently used: antipsychotics and benzodiazepines.
For agitation with perceptual disturbances associated with sleep-wake cycle abnormalities and uncontrolled behavior problems, antipsychotic agents can be useful. Haloperidol, a typical antipsychotic, is commonly used to manage delirium in spite of weak evidence to support its efficacy and lack of U.S. Food and Drug Administration approval for this indication. It is preferred over other antipsychotics because of fewer anticholinergic and hypotensive side effects. However, the high potency of haloperidol is related to increased frequency of extrapyramidal side effects such as dystonic reactions, akathisia, tardive dyskinesia, and malignant catatonia [30]. Close observation of the patient is indispensable not only for effectiveness but also for the monitoring of adverse events. Haloperidol is also associated with increased risk of corrected Q-T interval prolongation, and electrocardiography monitoring is recommended after administration [30].
Due to the lower incidence of extrapyramidal side effects, atypical antipsychotics such as risperidone, olanzapine, and quetiapine have been more frequently used to manage delirium. Several recent studies have shown some promising results in both their efficacy and safety [77–79]. Studies have shown that there was no significant difference in the efficacy between haloperidol and atypical antipsychotics in the treatment of delirium [80,81].
In the treatment of delirium with antipsychotic medication, advanced age may be a predictor of poor response [81–84]. Compared with other antipsychotics, olanzapine may show poor response in patients of older age because of its high affinity for muscarinic receptors (Fig. 3) [81].
Benzodiazepines have also been used historically to sedate agitated patients with delirium. In light of evidence to suggest that benzodiazepines can increase both the risk and duration of delirium [85], particularly in the elderly, benzodiazepines should mainly be used for the management of agitation associated with sedative-hypnotic withdrawal (e.g., alcohol, benzodiazepines, barbiturates).
Postoperative delirium is a common complication and exerts an enormous burden on patients, their families, hospitals, and public resources. Its management should be maintained throughout all stages of surgery with regards to the following 3 aspects: prevention, assessment, and treatment. Systematic intervention should be implemented through a team-based multicomponent approach aimed to reduce the incidence and duration of delirium.
ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI16C0132).
References
1. Collinsworth AW, Priest EL, Campbell CR, Vasilevskis EE, Masica AL. A review of multifaceted care approaches for the prevention and mitigation of delirium in intensive care units. J Intensive Care Med. 2016; 31:127–41.

2. Aldecoa C, Bettelli G, Bilotta F, Sanders RD, Audisio R, Borozdina A, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017; 34:192–214.

3. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5Ⓡ). 5th ed. Arlington: American Psychiatric Association Publishing;2013. p. 596–601.
4. Olin K, Eriksdotter-Jönhagen M, Jansson A, Herrington MK, Kristiansson M, Permert J. Postoperative delirium in elderly patients after major abdominal surgery. Br J Surg. 2005; 92:1559–64.

5. Sharma PT, Sieber FE, Zakriya KJ, Pauldine RW, Gerold KB, Hang J, et al. Recovery room delirium predicts postoperative delirium after hip-fracture repair. Anesth Analg. 2005; 101:1215–20.

7. Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006; 96:747–53.

8. Meagher DJ, O'Hanlon D, O'Mahony E, Casey PR, Trzepacz PT. Relationship between symptoms and motoric subtype of delirium. J Neuropsychiatry Clin Neurosci. 2000; 12:51–6.

9. Robinson TN, Raeburn CD, Tran ZV, Brenner LA, Moss M. Motor subtypes of postoperative delirium in older adults. Arch Surg. 2011; 146:295–300.

10. Meagher D. Motor subtypes of delirium: past, present and future. Int Rev Psychiatry. 2009; 21:59–73.

11. O'Keeffe ST, Lavan JN. Clinical significance of delirium subtypes in older people. Age Ageing. 1999; 28:115–9.
12. Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006; 54:479–84.

13. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990; 113:941–8.
14. Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001; 29:1370–9.

15. Heo EY, Lee BJ, Hahm BJ, Song EH, Lee HA, Yoo CG, et al. Translation and validation of the Korean Confusion Assessment Method for the Intensive Care Unit. BMC Psychiatry. 2011; 11:94.

16. Neufeld KJ, Leoutsakos JS, Sieber FE, Joshi D, Wanamaker BL, Rios-Robles J, et al. Evaluation of two delirium screening tools for detecting post-operative delirium in the elderly. Br J Anaesth. 2013; 111:612–8.

17. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002; 166:1338–44.
18. European Delirium Association. The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med. 2014; 12:141.
19. Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The memorial delirium assessment scale. J Pain Symptom Manage. 1997; 13:128–37.

20. Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001; 13:229–42.
21. Lee Y, Ryu J, Lee J, Kim HJ, Shin IH, Kim JL, et al. Korean version of the delirium rating scale-revised-98: reliability and validity. Psychiatry Investig. 2011; 8:30–8.

22. Trogrlić Z, van der Jagt M, Bakker J, Balas MC, Ely EW, van der Voort PH, et al. A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Crit Care. 2015; 19:157.

23. American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015; 220:136–48.
24. Liu P, Li YW, Wang XS, Zou X, Zhang DZ, Wang DX, et al. High serum interleukin-6 level is associated with increased risk of delirium in elderly patients after noncardiac surgery: a prospective cohort study. Chin Med J (Engl). 2013; 126:3621–7.
25. Robinson TN, Raeburn CD, Angles EM, Moss M. Low tryptophan levels are associated with postoperative delirium in the elderly. Am J Surg. 2008; 196:670–4.

26. Cerejeira J, Batista P, Nogueira V, Vaz-Serra A, Mukaetova-Ladinska EB. The stress response to surgery and postoperative delirium: evidence of hypothalamic-pituitary-adrenal axis hyperresponsiveness and decreased suppression of the GH/IGF-1 Axis. J Geriatr Psychiatry Neurol. 2013; 26:185–94.
27. Chen XW, Shi JW, Yang PS, Wu ZQ. Preoperative plasma leptin levels predict delirium in elderly patients after hip fracture surgery. Peptides. 2014; 57:31–5.

28. Koponen H, Partanen J, Pääkkönen A, Mattila E, Riekkinen PJ. EEG spectral analysis in delirium. J Neurol Neurosurg Psychiatry. 1989; 52:980–5.

29. van der Kooi AW, Zaal IJ, Klijn FA, Koek HL, Meijer RC, Leijten FS, et al. Delirium detection using EEG: what and how to measure. Chest. 2015; 147:94–101.
30. Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock's Comprehensive Textbook of Psychiatry. 10th ed. Philadelphia: Wolters Kluwer;2017. p. 1177–89.
31. Choi SH, Lee H, Chung TS, Park KM, Jung YC, Kim SI, et al. Neural network functional connectivity during and after an episode of delirium. Am J Psychiatry. 2012; 169:498–507.

32. Kyeong S, Choi SH, Eun Shin J, Lee WS, Yang KH, Chung TS, et al. Functional connectivity of the circadian clock and neural substrates of sleep-wake disturbance in delirium. Psychiatry Res Neuroimaging. 2017; 264:10–2.

33. Pandharipande P, Shintani A, Peterson J, Pun BT, Wilkinson GR, Dittus RS, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006; 10:21–6.

34. Park S, Na SH, Oh J, Lee JS, Oh ST, Kim JJ, et al. Pain and anxiety and their relationship with medication doses in the intensive care unit. J Crit Care. 2018; 47:65–9.

35. Zaal IJ, Spruyt CF, Peelen LM, van Eijk MM, Wientjes R, Schneider MM, et al. Intensive care unit environment may affect the course of delirium. Intensive Care Med. 2013; 39:481–8.

36. Barr J, Donner A. Optimal intravenous dosing strategies for sedatives and analgesics in the intensive care unit. Crit Care Clin. 1995; 11:827–47.

37. Abelha FJ, Fernandes V, Botelho M, Santos P, Santos A, Machado JC, et al. Apolipoprotein E e4 allele does not increase the risk of early postoperative delirium after major surgery. J Anesth. 2012; 26:412–21.

38. Ansaloni L, Catena F, Chattat R, Fortuna D, Franceschi C, Mascitti P, et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg. 2010; 97:273–80.

39. Nadelson MR, Sanders RD, Avidan MS. Perioperative cognitive trajectory in adults. Br J Anaesth. 2014; 112:440–51.

40. Jia Y, Jin G, Guo S, Gu B, Jin Z, Gao X, et al. Fast-track surgery decreases the incidence of postoperative delirium and other complications in elderly patients with colorectal carcinoma. Langenbecks Arch Surg. 2014; 399:77–84.

41. Xará D, Silva A, Mendonça J, Abelha F. Inadequate emergence after anesthesia: emergence delirium and hypoactive emergence in the Postanesthesia Care Unit. J Clin Anesth. 2013; 25:439–46.

42. Krzych LJ, Wybraniec MT, Krupka-Matuszczyk I, Skrzypek M, Bolkowska A, Wilczyński M, et al. Complex assessment of the incidence and risk factors of delirium in a large cohort of cardiac surgery patients: a single-center 6-year experience. Biomed Res Int. 2013; 2013:835850.

43. Krzych LJ, Wybraniec MT, Krupka-Matuszczyk I, Skrzypek M, Bochenek AA. Delirium Screening in Cardiac Surgery (DESCARD): a useful tool for nonpsychiatrists. Can J Cardiol. 2014; 30:932–9.

44. Haenggi M, Blum S, Brechbuehl R, Brunello A, Jakob SM, Takala J. Effect of sedation level on the prevalence of delirium when assessed with CAM-ICU and ICDSC. Intensive Care Med. 2013; 39:2171–9.

45. Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014; 189:658–65.

46. Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med. 2014; 370:444–54.

47. Shin JE, Kyeong S, Lee JS, Park JY, Lee WS, Kim JJ, et al. A personality trait contributes to the occurrence of postoperative delirium: a prospective study. BMC Psychiatry. 2016; 16:371.

48. Lum PY, Singh G, Lehman A, Ishkanov T, Vejdemo-Johansson M, Alagappan M, et al. Extracting insights from the shape of complex data using topology. Sci Rep. 2013; 3:1236.

49. Goldberg LR. An alternative "description of personality": the big-five factor structure. J Pers SocPsychol. 1990; 59:1216–29.

50. McNiel JM, Fleeson W. The causal effects of extraversion on positive affect and neuroticism on negative affect: Manipulating state extraversion and state neuroticism in an experimental approach. J Res Personal. 2006; 40:529–50.

51. Larsen RJ, Ketelaar T. Personality and susceptibility to positive and negative emotional states. J Pers Soc Psychol. 1991; 61:132–40.

52. Connor-Smith JK, Flachsbart C. Relations between personality and coping: a meta-analysis. J Pers Soc Psychol. 2007; 93:1080–107.

53. Jensen-Campbell LA, Knack JM, Waldrip AM, Campbell SD. Do Big Five personality traits associated with self-control influence the regulation of anger and aggression? J Res Personal. 2007; 41:403–24.

54. Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012; 367:30–9.
55. Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009; 180:1092–7.

56. Duppils GS, Wikblad K. Cognitive function and health-related quality of life after delirium in connection with hip surgery. A six-month follow-up. Orthop Nurs. 2004; 23:195–203.
57. Balas MC, Happ MB, Yang W, Chelluri L, Richmond T. Outcomes associated with delirium in older patients in surgical ICUs. Chest. 2009; 135:18–25.

58. Sohn JH, Na SH, Shin CS, Sohn I, Oh JY, An JS, et al. Impact of delirium on clinical outcomes in intensive care unit patients: an observational study in a Korean General Hospital. J Korean Neuropsychiat Assoc. 2014; 53:418–25.

59. Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001; 27:1892–900.

60. Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013; 41:263–306.

61. Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004; 291:1753–62.

62. Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009; 249:173–8.
63. Marcantonio ER, Goldman L, Mangione CM, Ludwig LE, Muraca B, Haslauer CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994; 271:134–9.

64. Markar SR, Smith IA, Karthikesalingam A, Low DE. The clinical and economic costs of delirium after surgical resection for esophageal malignancy. Ann Surg. 2013; 258:77–81.

65. Bellelli G, Mazzola P, Morandi A, Bruni A, Carnevali L, Corsi M, et al. Duration of postoperative delirium is an independent predictor of 6-month mortality in older adults after hip fracture. J Am Geriatr Soc. 2014; 62:1335–40.

66. Krzych LJ, Wybraniec MT, Krupka-Matuszczyk I, Skrzypek M, Bolkowska A, Wilczyński M, et al. Detailed insight into the impact of postoperative neuropsychiatric complications on mortality in a cohort of cardiac surgery subjects: a 23,000-patient-year analysis. J Cardiothorac Vasc Anesth. 2014; 28:448–57.

67. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010; 304:443–51.
68. Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013; 369:1306–16.

69. Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008; 108:18–30.

70. Lundström M, Edlund A, Bucht G, Karlsson S, Gustafson Y. Dementia after delirium in patients with femoral neck fractures. J Am Geriatr Soc. 2003; 51:1002–6.
71. Gruber-Baldini AL, Zimmerman S, Morrison RS, Grattan LM, Hebel JR, Dolan MM, et al. Cognitive impairment in hip fracture patients: timing of detection and longitudinal follow-up. J Am Geriatr Soc. 2003; 51:1227–36.

72. Edlund A, Lundström M, Brännström B, Bucht G, Gustafson Y. Delirium before and after operation for femoral neck fracture. J Am Geriatr Soc. 2001; 49:1335–40.

73. Kim S, Kim JJ, Oh J, Park J, Park JY. Delirium characteristics and outcomes in medical and surgical lnpatients: a subgroup analysis. J Crit Care. 2018; 43:156–62.

74. Heymann A, Radtke F, Schiemann A, Lütz A, MacGuill M, Wernecke KD, et al. Delayed treatment of delirium increases mortality rate in intensive care unit patients. J Int Med Res. 2010; 38:1584–95.

75. Inouye SK, Bogardus ST Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999; 340:669–76.

76. Hshieh TT, Yue J, Oh E, Puelle M, Dowal S, Travison T, et al. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015; 175:512–20.
77. Schwartz TL, Masand PS. The role of atypical antipsychotics in the treatment of delirium. Psychosomatics. 2002; 43:171–4.

78. Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004; 30:444–9.

79. Pae CU, Lee SJ, Lee CU, Lee C, Paik IH. A pilot trial of quetiapine for the treatment of patients with delirium. Hum Psychopharmacol. 2004; 19:125–7.

80. Grover S, Kumar V, Chakrabarti S. Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. 2011; 71:277–81.

81. Yoon HJ, Park KM, Choi WJ, Choi SH, Park JY, Kim JJ, et al. Efficacy and safety of haloperidol versus atypical antipsychotic medications in the treatment of delirium. BMC Psychiatry. 2013; 13:240.

82. Kim SW, Yoo JA, Lee SY, Kim SY, Bae KY, Yang SJ, et al. Risperidone versus olanzapine for the treatment of delirium. Hum Psychopharmacol. 2010; 25:298–302.

83. Breitbart W, Tremblay A, Gibson C. An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics. 2002; 43:175–82.

84. Lonergan E, Britton AM, Luxenberg J, Wyller T. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007; (2):CD005594.

85. Gaudreau JD, Gagnon P. Psychotogenic drugs and delirium pathogenesis: the central role of the thalamus. Med Hypotheses. 2005; 64:471–5.

Fig. 1.
Topological data analysis of patient-patient networks for psychological risk factors in postoperative delirium. Filter metric was subdivided into 8 intervals with 80% overlap. Several nodes were disconnected from the main graph. An inset graph in the bottom right represents a lower resolution topology with 4 intervals and 60% overlap. Subgroup G1 includes 7 delirious patients with low Mini-Mental State Examination (MMSE) scores and regional anesthesia and G2 includes 4 delirious patients with medium MMSE scores, high neuroticism, and low conscientiousness scores. G0 includes 6 patients with high MMSE, low neuroticism, and high conscientiousness scores. Adapted from Shin et al. [47] with permission.
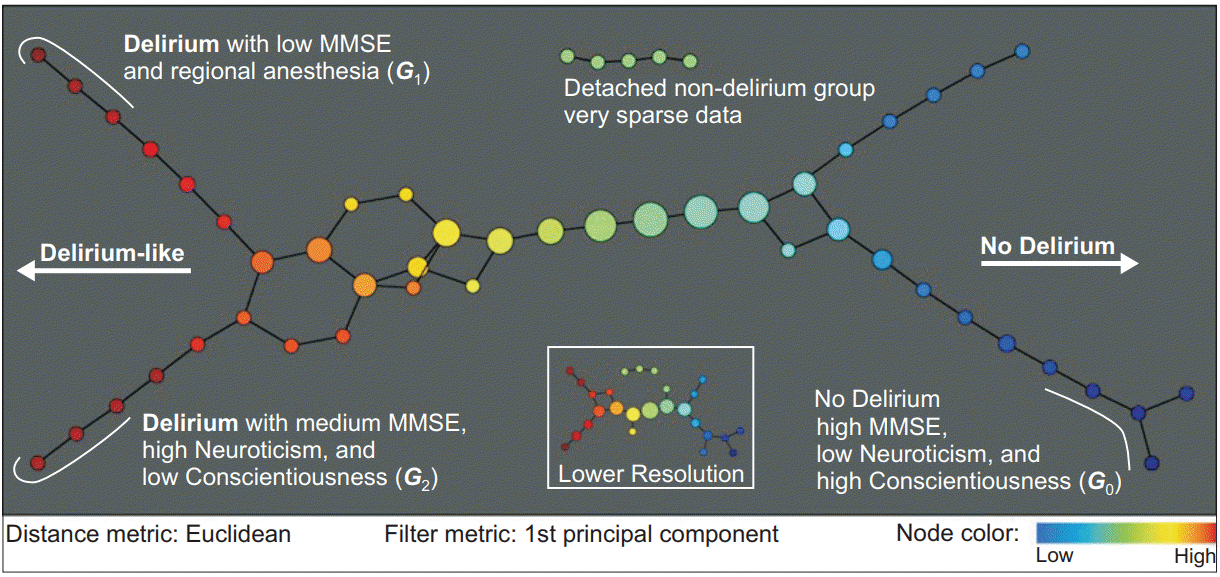
Fig. 2.
Delirium recovery rate according to the time course. The graph shows the time course of delirium recovery among 88 patients whose delirium was resolved during hospitalization (72%). The proportion of patients with delirium decreased with increasing length of delirium duration. A total of 39 days were required for medical patients to recover, versus 16 days and 77 days for postoperative and postoperativedelayed patients who recovered during hospitalization, respectively. Adapted from Kim et al. [73] with permission.
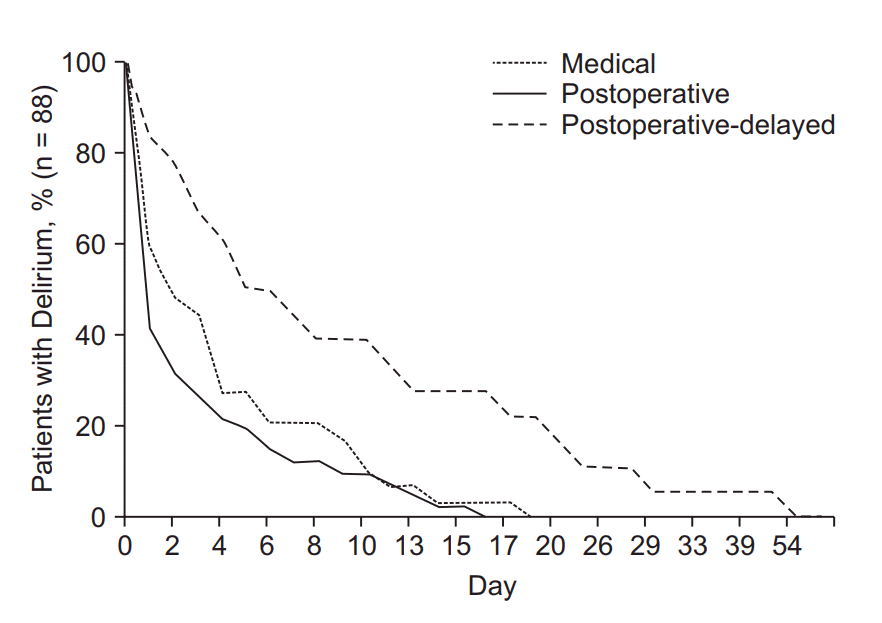
Fig. 3.
Treatment response rate between young-old and old-old groups in the 4 antipsychotic groups. *P < 0.05 by Chi-square test or Fisher’s exact test. Treatment response was defined as a ≥ 50% reduction from the baseline score on the Korean version of the Delirium Rating ScaleRevised-98 (DRS-R98-K) [21]. DRS-R98-K is evaluates the presence and severity of delirium. This figure is adapted from Yoon et al. [81] with permission.
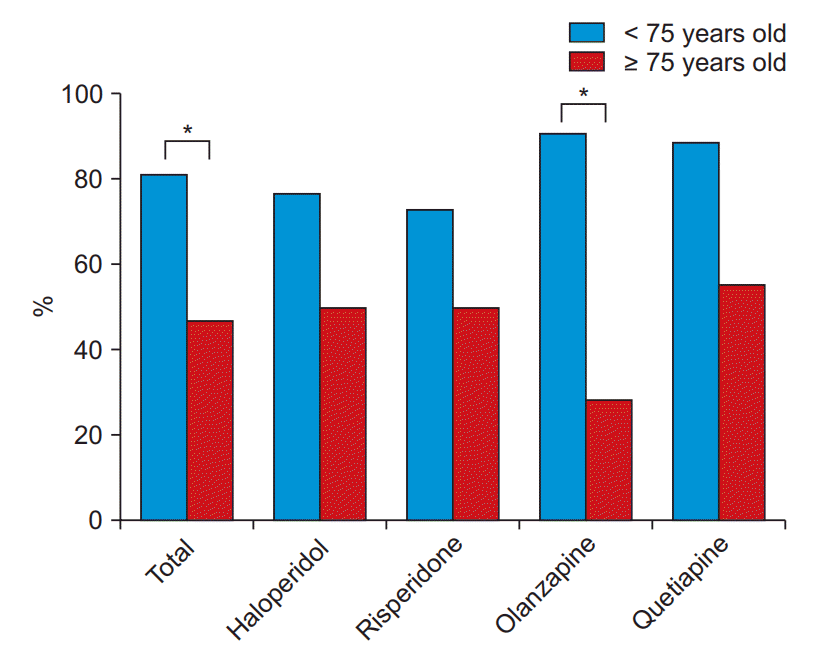
Table 1.
Definition of Delirium by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [3]
Table 2.
Risk Factors of Postoperative Delirium [2]




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download