Abstract
Background
Cerebral state index (CSI) is an anesthesia depth monitor alternative to bispectral index (BIS). Published comparative studies have used propofol or sevoflurane. However, studies using desflurane have not been reported yet. Different volatile anesthetics have different electroencephalography signatures. The performance of CSI may be different in desflurane anesthesia. Therefore, the objective of this study was to compare CSI and BIS during desflurane anesthesia.
Methods
Thirty-three patients were recruited. Desflurane and remifentanil were used to maintain general anesthesia. BIS and CSI were recorded simultaneously every minute. End-tidal concentration of desflurane was maintained at 4% from the beginning of surgery for 5 minutes. Pairwise data of CSI and BIS were obtained five times at one-minute intervals. This process was repeated in the order of 6%, 8%, and 10%.
Results
BIS and CSI were negatively correlated with the end-tidal concentration of desflurane with a similar degree of correlation (correlation coefficient BIS: –0.847, CSI: –0.844). The relationship between CSI and BIS had a good linearity with a slope close to 1 (R2 = 0.905, slope = 1.01). For the relationship between CSI and BIS at each end-tidal concentration of desflurane, CSI and BIS showed good linearity in 4% and 10% (R2 = 0.559, 0.540). However, the linearity and slope were decreased in 6% and 8% (R2 = 0.163, 0.014).
Monitors for the depth of anesthesia based on electroencephalography (EEG) have been studied and many indices have been developed and commercialized. These kinds of monitoring have been widely used because they can prevent complications and save costs from excessive anesthesia. They can also prevent awareness during light anesthesia [1]. Recently, bispectral index (BIS) as a golden standard for anesthesia depth monitoring is most commonly used. However, it has been reported that the incidence of awareness is not lowered by maintaining BIS value below 60 [2,3]. In addition, the high cost of BIS makes us question the usefulness of BIS for the general population.
Similar to BIS, cerebral state index (CSI) is a monitor for determining the depth of anesthesia using a single channel EEG. These two indices are scaled from 0 (isoelectric EEG) to 100 (full awake). During general anesthesia, it is recommended that BIS and CSI should be maintained between 40 and 60. CSI uses an algorithm different from that used for BIS. It has four sub-parameters derived from time domain analysis (burst-ratio) and frequency domain analysis (α-ratio, β-ratio, and β-ratio-α-ratio) of EEG [4].
Clinical advantages of CSI includes its cost-effectiveness and portability [1,5]. Portability makes CSI a good alternative to BIS in anesthesia, especially outside the operating room such as the endoscopy room and angiography room. Cost-effectiveness is especially beneficial for small sized hospitals where anesthesia equipment is not abundant. CSI has an additional advantage in that it causes no pain when applying the sensor.
Several previous studies have demonstrated the correlation between CSI and BIS. However, propofol or sevoflurane was used in most previous studies on the correlation between CSI and BIS [6]. Studies on the CSI and BIS correlation using desflurane have not been reported yet. Desflurane is a commonly used volatile anesthetic. It has the advantages of rapid induction and rapid emergence from anesthesia. However, different inhaled anesthetics have different EEG signatures [3]. It is known that values of BIS can be different for an equipotent concentration of different inhaled anesthetics [3]. Thus, the performance of CSI might be different in desflurane anesthesia. Therefore, the purpose of this study was to compare CSI and BIS in general anesthesia using desflurane and remifentanil. Correlations between the end-tidal concentration of desflurane and BIS and CSI were then quantified.
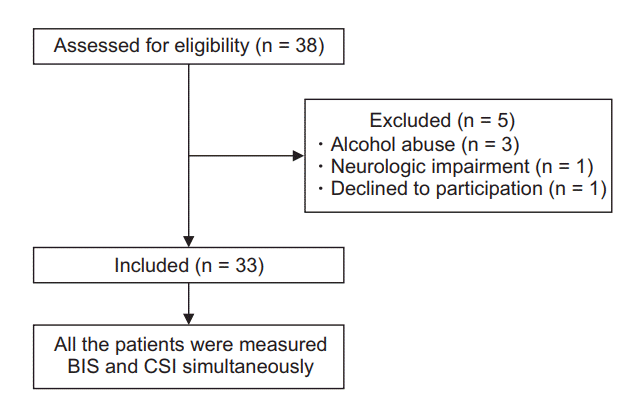
After obtaining approval from the Institutional Review Board of our Hospital (IRB number: 2017-07-005), 33 patients receiving abdominal surgery were recruited after explaining the purpose of this study and obtaining consent from each patient. The sample size was determined to apply parametric statistics. Patients aged 18 to 75 years who were classified as American Society of Anesthesiologists (ASA) class I or II (ASA I: a normal healthy patient; ASA II: a patient with mild systemic disease) were included. Exclusion criteria were: 1) neurologic impairment, 2) medications that might affect cerebral functions such as hypnotics and antidepressants, 3) history of alcohol abuse, and 4) patients with mental retardation.
Patients were transferred to the operating room without premedication. After applying standard monitoring, the skin for the sensor attachment area was cleaned with alcohol and then dried. BIS (Aspect Medical Systems, Inc., USA) and CSI (Chengdu MaiGao Medical Equipment Co., China) sensors were attached simultaneously to the ipsilateral forehead. Pairwise data of CSI and BIS were recorded every minute. Baseline BIS and CSI values were measured before initiating general anesthesia. Induction of anesthesia was then started by injecting pentothal sodium (4–5 mg/kg), rocuronium (0.6 mg/kg). Ninety percent O2 and 10% desflurane (flow = 8 L/min) were applied to the patient, remifentanil was infused at 0.2 μg/kg/min to facilitate intubation. A circle breathing system ventilator (Primus, Dragger, Germany) was used during anesthesia. Desflurane and infusion of remifentanil (0.05–0.20 μg/kg/min) were used to maintain general anesthesia. Additional rocuronium was injected when necessary.
After the operation was started, the end-tidal concentration of desflurane was maintained at 4% for 5 minutes (mixed gas of O2 and air, FiO2 = 50%, flow = 5 L/min). The fresh gas flow was then lowered to 3 L/min. BIS and CSI values were measured five times at one-minute intervals simultaneously. This process was repeated in the order of 6%, 8%, and 10% of the end-tidal concentration of desflurane. At the end of the operation, desflurane was removed with 100% oxygen (flow = 8 L/min). Reversal agents (15 mg of pyridostigmine and 0.4 mg of glycopyrrolate) were then injected. Removal of endotracheal tube was performed when patient sustained five-second head-tilt or hand grasp and obeyed a command.
Data were expressed as means ± SD for normally distributed continuous variables and median (25%–75%) for non-normally distributed continuous variables. Pearson’s correlation analysis was performed to determine the correlation according to the end-tidal concentration of desflurane. Linear regression analysis was used to determine the relationship between BIS and CSI. Bland-Altman plot was used to study the difference (BIS-CSI) and limits of agreement (difference ± 2SD). Multiple comparisons between these indices were performed with repeated measures analysis of variance (ANOVA). P value < 0.05 was considered statistically significant. SPSS version 23.0 (IBM Corp., USA) was used for all perform statistical analyses.
Thirty-three patients who met the inclusion criteria completed this study (Fig. 1). There were 21 males and 12 females. Their mean age, weight, and height were 54.5 ± 13.5 years, 66.9 ± 14.5 kg, and 164.2 ± 10.0 cm, respectively (mean ± SD). Surgeries included cholecystectomy (n = 18), gastrectomy (n = 5), appendectomy (n = 8), ileostomy repair (n = 1), and herniorraphy (n = 1).
Overall BIS and CSI progressively decreased with increasing, end-tidal concentration of desflurane (Fig. 2). Results of Pearsons correlation analysis for the two indices according to the end-tidal concentration of desflurane are shown in Table 1. There was no significant difference in the correlation between BIS and CSI according to the end-tidal concentration of desflurane.
The correlation between BIS and CSI as a scatter plot is shown in Fig. 3. The relationship between the two indices through linear regression analysis was ‘CSI = 1.01 × BIS + 1.9’ (P < 0.001, R2 = 0.905). In Fig. 4, the relationship between BIS and CSI at each end-tidal concentration of desflurane (4%, 6%, 8%, and 10%) is plotted. The two indices showed linear correlations with R2 = 0.559 and R2 = 0.540 when the end-tidal concentration of desflurane was 4% and 10%, respectively. But the linearity was relatively low when the end-tidal concentration of desflurane was 6% and 8% (R2 = 0.014 and 0.163, respectively).
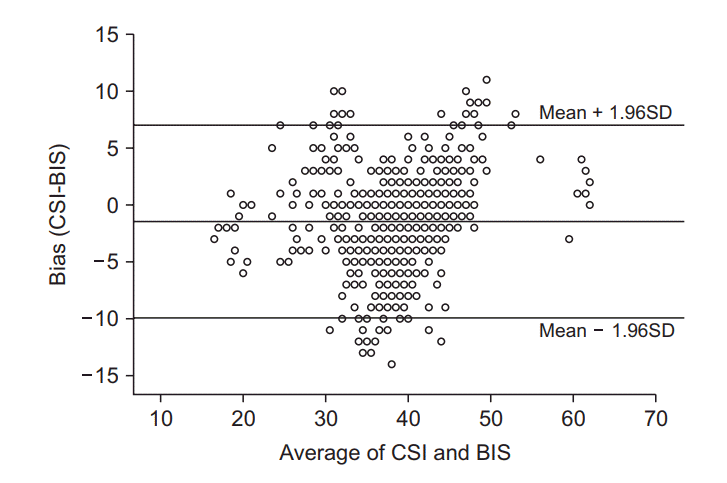
The difference between the two indices is shown through a Bland-Altman plot (Fig. 5). This graph shows the tendency of the difference between CSI and BIS according to the average value of the two parameters. The difference between the two parameters did not show a specific tendency in the whole range. Most values were included in the 95% confidence interval.
The result of the repeated measures ANOVA revealed that both indices showed statistically significant changes with respect to the end-tidal concentration of desflurane (P < 0.001). BIS and CSI values showed significant differences according to the end-tidal concentration of desflurane (P < 0.001).
In this study, we determined the relationship between BIS and CSI for patients undergoing abdominal surgery using desflurane for anesthesia. Our results demonstrated that CSI and BIS only had minor differences in dose-response relations, although their algorithms had major differences. Overall, BIS and CSI progressively decreased with increasing end-tidal concentration of desflurane, but a prominent plateau was observed in most cases of CSI.
The algorithm used for CSI was Adaptive Neuro-Fuzzy Inference System (ANFIS) [7]. At first, the ANFIS obtained the algorithm with patients using propofol and patients using sevoflurane [7]. Subsequent comparative studies between BIS and CSI mostly used sevoflurane or propofol. However, different volatile anesthetic agents have different EEG signatures [3]. For example, the clinical concentration of halothane produces slow waves and fast spindle EEG rhythms whereas sevoflurane produces sharp and slow EEG waves with relatively few fast rhythms [3]. In addition, it is known that values of BIS can be different for an equipotent concentration of different inhaled anesthetics. For example, BIS values during halothane anesthesia are higher than those during equipotent sevoflurane, isoflurane, and desflurane anesthesia [8]. Desflurane produces a greater hypnotic effect than sevoflurane during equipotent anesthesia [9]. Thus, we thought that CSI might show a different performance during desflurane anesthesia. Therefore, this study was designed to compare BIS and CSI during desflurane anesthesia.
Remifentanil was used in addition to desflurane in this study. Noncortical structures are involved in the mechanism of action of remifentanil, different from intravenous and inhalational agents [10]. Thus, remifentanil cannot change the cerebral cortical EEG significantly. It has been proven in a previous study that high dose of remifentanil during continuous infusion with propofol does not change BIS [11]. Therefore, we used remifentanil to minimize the effect of surgical stimulation while minimizing its effect on BIS and CSI.
There was no significant difference in overall performance between CSI and BIS. However, the difference between CSI and BIS was increased when the end-tidal concentration of desflurane was 6% and 8%. Linearity and agreement between BIS and CSI were also decreased in this concentration range of desflurane. In our study, the value of CSI showed stabilization around 40 and a plateau was observed. The relationship between CSI and BIS showed a slope of 0.22 and 0.09 for the end-tidal concentration of desflurane at 6% and 8%, respectively. This means that CSI has changed little with a change in BIS. Thus, we assume that such a plateau can increase the pair-wise difference between CSI and BIS. But this result does not correspond to the study conducted by Nishiyama [6] comparing BIS and CSI in general anesthesia using sevoflurane and nitrous oxide. In that study, BIS and CSI showed good linear correlations. When the end-tidal concentration of sevoflurane was 0.5%, 1%, and 1.5%, R2 values were 0.803, 0.934, and 0.878, respectively (slopes of 0.966, 0.966, and 0.749, respectively). However, in other several comparative studies, stabilization of CSI is often observed and referred to as a feature of CSI [12-14].
However, in deep anesthesia with an end-tidal concentration of desflurane at 10%, the linearity and agreement between BIS and CSI were improved than those at 6% or 8%. The EEG pattern known as burst suppression appeared in deep general anesthesia. This pattern is characterized by alternating periods of normal to high voltage activity changing to low voltage or even isoelectricity [15]. BIS and CSI commonly use burst suppression ratio (BSR) as a parameter. In our study, as BSR values of both indices increased in deep general anesthesia, both indices decreased with high correlation. According to Cortínez et al. [14], CSI is more suitable for deep anesthesia because it is highly correlated with BSR.
In light anesthesia with an end-tidal concentration of desflurane at 4%, BIS and CSI values also showed good linearity and agreement. β and α waves are dominant EEG signals in light anesthesia and they are aroused from the cortical brain. As the depth of anesthesia increases, EEG slows down toward θ and δ waves originating in the deep brain [3,16]. BIS is linearly correlated with relative β ratio when BIS is higher than 60 [17]. CSI uses α-ratio, β-ratio, and β-ratio-α-ratio as parameters too. Thus, we assumed that the use of two indices as a parameter of α and β wave makes good agreement and correlation in light anesthesia.
In this study, CSI showed a narrow variation. It was more stable compared to BIS. The standard deviation of CSI measured at one-minute intervals was statistically significantly lower than that of BIS (P < 0.05). Thus, CSI might be more stable than BIS when the anesthetic agent was maintained constant. In a previous study, CSI has a less dynamic profile compared to BIS in moderate anesthetic depth. Delfino et al. [18] have explained that such a less dynamic profile of CSI might be because CSI’s algorithm does not include frequencies below 6 Hz that are found in δ and θ waves. CSI was less sensitive until much deeper hypnotic levels (marked by the start of burst suppression activity) are reached.
In conclusion, this study demonstrates that CSI has an equivalent degree of overall performance compared to BIS in general anesthesia using desflurane. Therefore, accounting for the avidence from previous Literature, CSI can be used as a good substitute for BIS regardless of the kind of common anesthetics currently in use.
References
2. Avidan MS, Zhang L, Burnside BA, Finkel KJ, Searleman AC, Selvidge JA, et al. Anesthesia awareness and the bispectral index. N Engl J Med. 2008; 358:1097–108.

3. Dahaba AA. Different conditions that could result in the bispectral index indicating an incorrect hypnotic state. Anesth Analg. 2005; 101:765–73.

4. Han DW, Linares-Perdomo OJ, Lee JS, Kim JH, Kern SE. Comparison between cerebral state index and bispectral index as measures of electroencephalographic effects of sevoflurane using combined sigmoidal E(max) model. Acta Pharmacol Sin. 2011; 32:1208–14.

5. Hoymork SC, Hval K, Jensen EW, Raeder J. Can the cerebral state monitor replace the bispectral index in monitoring hypnotic effect during propofol/remifentanil anaesthesia? Acta Anaesthesiol Scand. 2007; 51:210–6.

6. Nishiyama T. Cerebral state index vs. bispectral index during sevoflurane-nitrous oxide anaesthesia. Eur J Anaesthesiol. 2009; 26:638–42.

7. Jensen EW, Litvan H, Revuelta M, Rodriguez BE, Caminal P, Martinez P, et al. Cerebral state index during propofol anesthesia: a comparison with the bispectral index and the A-line ARX index. Anesthesiology. 2006; 105:28–36.
8. Edwards JJ, Soto RG, Thrush DM, Bedford RF. Bispectral index scale is higher for halothane than sevoflurane during intraoperative anesthesia. Anesthesiology. 2003; 99:1453–5.

9. Kim JK, Kim DK, Lee MJ. Relationship of bispectral index to minimum alveolar concentration during isoflurane, sevoflurane or desflurane anaesthesia. J Int Med Res. 2014; 42:130–7.

10. Pan YZ, Li DP, Chen SR, Pan HL. Activation of mu-opioid receptors excites a population of locus coeruleus-spinal neurons through presynaptic disinhibition. Brain Res. 2004; 997:67–78.
11. Guignard B, Menigaux C, Dupont X, Fletcher D, Chauvin M. The effect of remifentanil on the bispectral index change and hemodynamic responses after orotracheal intubation. Anesth Analg. 2000; 90:161–7.

12. Fuentes R, Cortínez LI, Struys MM, Delfino A, Muñoz H. The dynamic relationship between end-tidal sevoflurane concentrations, bispectral index, and cerebral state index in children. Anesth Analg. 2008; 107:1573–8.

13. Anderson RE, Jakobsson JG. Cerebral state monitor, a new small handheld EEG monitor for determining depth of anaesthesia: a clinical comparison with the bispectral index during day-surgery. Eur J Anaesthesiol. 2006; 23:208–12.
14. Cortínez LI, Delfino AE, Fuentes R, Muñoz HR. Performance of the cerebral state index during increasing levels of propofol anesthesia: a comparison with the bispectral index. Anesth Analg. 2007; 104:605–10.
16. Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010; 363:2638–50.

Fig. 2.
Changes in the bispectral index (BIS) and cerebral state index (CSI) according to the end-tidal concentration of desflurane are shown as medians with first and third quartiles (box) and maximum and minimum values. Pre: pre-anesthesia, Post: post-extubation.
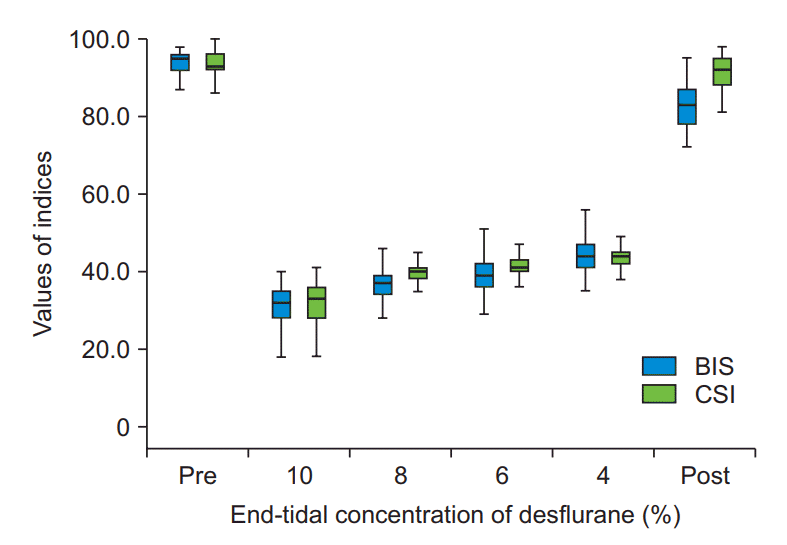
Fig. 3.
The scattered plot of the simultaneously measured bispectral index (BIS) and cerebral state index (CSI) in all anesthesia depths. The coefficient of determination denoted R2 is the proportion of the variance in CSI that is predictable from BIS. The solid lines are linear regression lines.
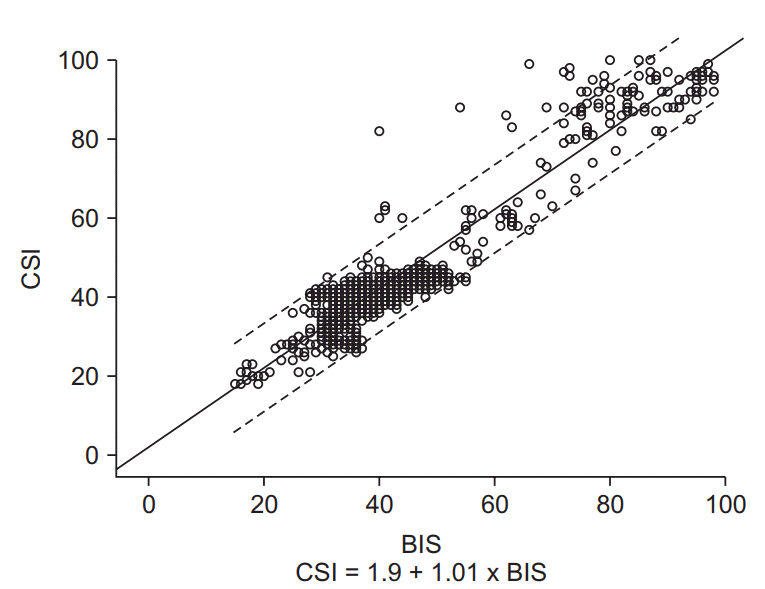
Fig. 4.
The scattered plot of the simultaneously measured bispectral index (BIS) and cerebral state index (CSI) at each end-tidal concentration of desflurane. (A) End-tidal concentration of desflurane at 4%, (B) End-tidal concentration of desflurane at 6%, (C) End-tidal concentration of desflurane at 8%, (D) End-tidal concentration of desflurane at 10%.
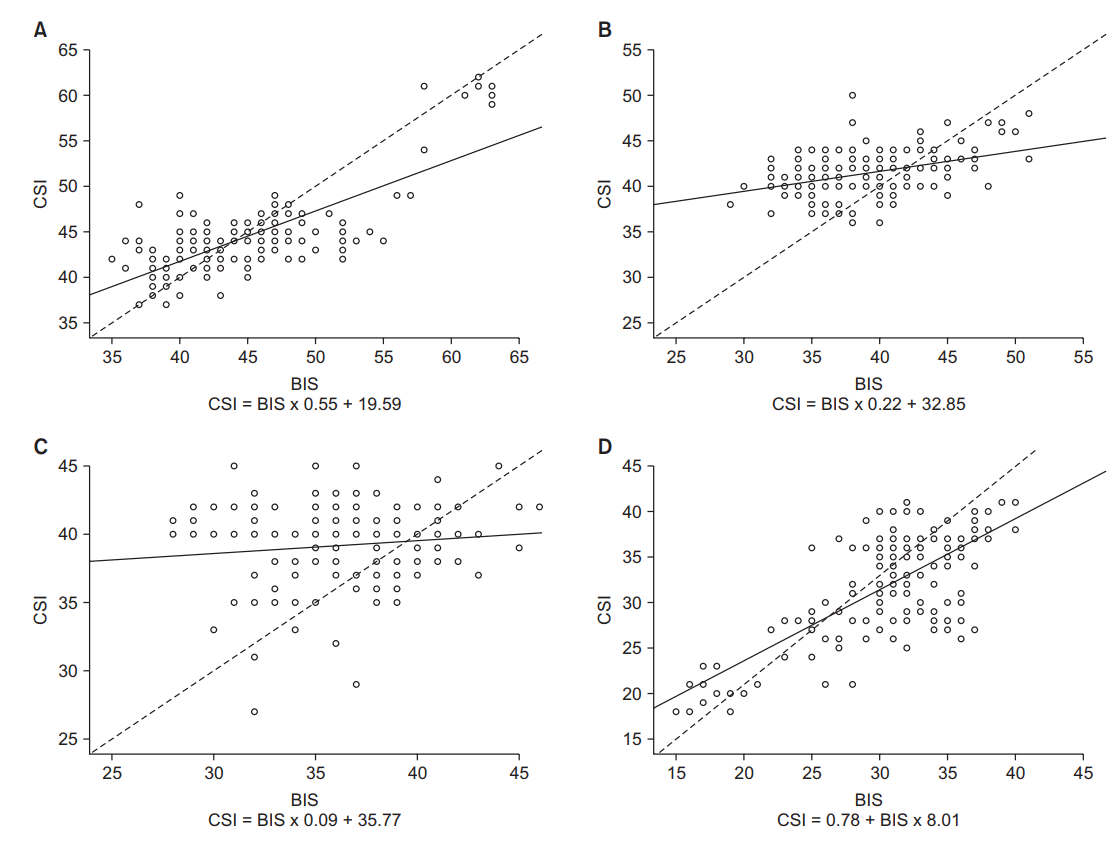
Fig. 5.
Bland-Altman plots. Showing the differences between bispectral index (BIS) and cerebral state index (CSI) according to the level of the average values of CSI and BIS. The solid line indicates the mean difference. Dotted lines are the mean difference plus and minus 1.96 times of the standard deviation of differences (95% CI). The mean difference between BIS and CSI (BIS-CSI) was –1.92 with 95% limits of agreement of 8.076 and –11.916.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download