Abstract
Background
Woake’s syndrome (WS) is a recurrent nasal polyposis, accompanied by broadening of the nose, frontal sinus aplasia, dyscrinia, and bronchiectasis. There has been no previous report on anesthetic management in patients with WS.
Case
We describe a case involving a 13-year-old male patient with WS who was scheduled for septorhinoplasty for necrotic ethmoiditis. Anesthesia was induced and maintained with propofol and remifentanil using a target-controlled infusion device. The anesthetic considerations of this rare syndrome and the advantages of an intravenous infusion method over local and volatile anesthesia for these patients are discussed. We report on caveats, such as pulmonary dysfunction during the anesthetic management, and nasal structural problems encountered in WS patients.
Conclusions
Given that conventional inhaled anesthesia reduces ciliary movement and that local anesthesia with sedative has several disadvantages, perioperative control and precautions against respiratory infections by using antibiotics, and preventing cilio-depressant actions, are important for anesthetic management.
Woake’s syndrome (WS) was first reported in 1885, characterized by necrotizing ethmoiditis, recurrent nasal polyposis, with consecutive destruction of the nasal pyramid, leading to broadening of the nose due to the chronic pressure from the polyps, frontal sinus aplasia, dyscrinia, and bronchiectasis [1]. Nasal polyps, which develop during childhood, are the primary characteristics of this rare condition. Causing severe nasal deformity and necrosis of the star-shaped bone cells due to extensive polyp growth in the paranasal sinuses and nasal cavity. Patients with WS also complain about abundant nasal discharge of rubber-like secretions, without any sign of allergies [2].
The pathophysiological process underlying the inflammation that leads to chronic hyper proliferative sinusitis and nasal polyposis and the pathoetiology of this syndrome are still unclear [3]. Groman et al. [4] have suggested that genetic factors are more common among siblings. They concluded that severe sinus disease seen in these siblings has causes other than the known autosomal recessive diseases associated with recurrent and destructive nasal polyposis. External harmful substances and allergies can accelerate the growth of polyps. However, in many cases of WS, no agents or allergies were found, indicating that this syndrome, involving deformed and recurrent polyps, is a separate clinical entity. The extreme expansion of the nose is explained as a consequence of the chronic pressure of the polyps.
WS patients with bronchiectasis have been treated effectively using physiotherapy and thorough suctioning before and during anesthesia, which prevents obstruction of the respiratory tract and improves respiratory sound after surgery [5]. Moreover, given that upper and lower airway ciliary function is important for pulmonary defense and that conventional inhaled anesthesia reduces ciliary movement, this form of anesthesia could be particularly problematic for patients with WS [6–8]. Preservation of mucociliary movement by using intravenous anesthesia involving propofol and remifentanil may, therefore, benefit WS patients that have pulmonary problems.
Therefore, anesthetic management of patients with WS should be considered carefully. Herein, we describe a case of successful anesthetic management of a young patient with WS who underwent both endoscopic sinus surgery (ESS) and septorhinoplasty.
A 13-year-old male (height: 143 cm; weight: 30 kg) patient presented for surgery with recurring complaints of frequent nasal obstruction, congestion, hyposmia, rhinorrhea, epistaxis, postnasal drip, headache, and snoring since the age of 5 years. Physical examination revealed nose enlargement with no distinctive widening of the nasal bones and a polypoid mass filling the entire nasal cavity. Our case history as well as clinical and laboratory findings fit neither the diagnosis of cystic fibrosis nor that of Kartagener’s syndrome; a differential diagnosis of those syndromes was performed at the Department of Otorhinolaryngology of our institute. Atopic disease was excluded by extensive testing, and ‘asthma, aspirin intolerance, and nasal polyps’ disease was excluded by the absence of asthma, a lack of a family history of aspirin intolerance, and by the early onset of polyposis. Once bronchiectasis and dyscrinia were detected in this patient, a diagnosis of WS was made.
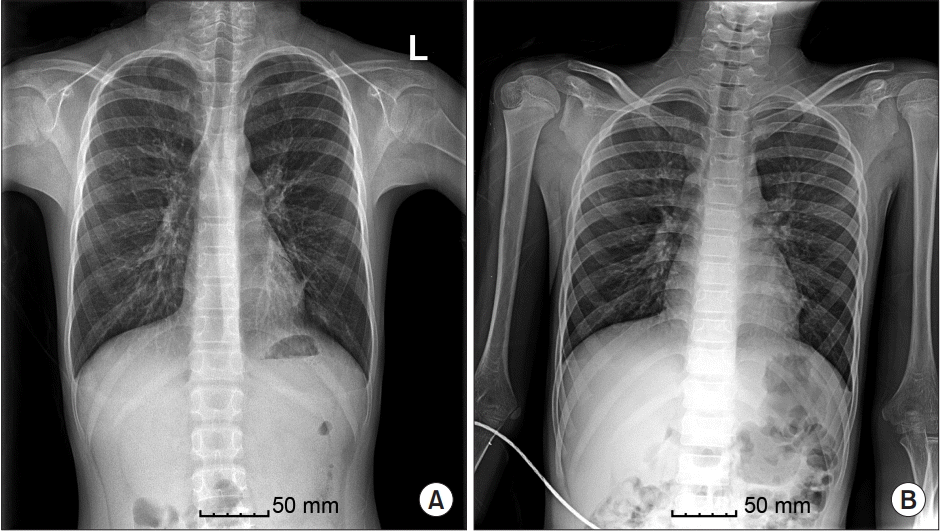
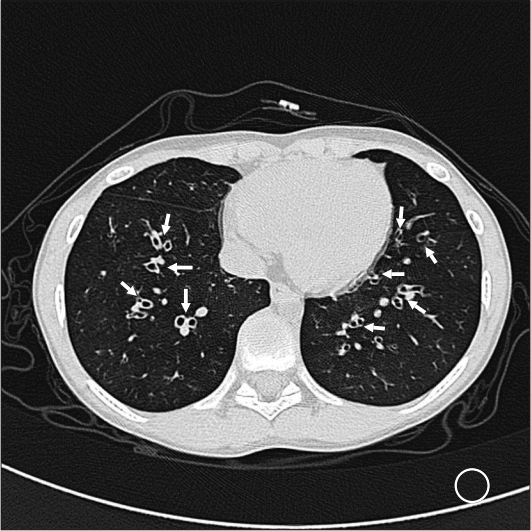
After medical treatment with topical and systemic steroids failed, the patient underwent bilateral nasal polyp removal surgery for the first time at the age of 6 years. At 7, 8, and 10 years of age, he had undergone both ESS and polypectomy elsewhere, and no bronchiectasis was found prior to surgery. Both ESS and septorhinoplasty were also planned for the surgery at our institution. During preoperative evaluation, chest radiography ruled out interstitial pneumonia in both lungs and in the left retrocardiac area (Fig. 1A). High-resolution computed tomography of the chest revealed diffuse bronchial dilatation with bronchial wall thickening and distal air trapping in both lungs (Fig. 2), as well as bronchial asthma or ciliary dyskinesia syndrome. Spirometry revealed a forced vital capacity (FVC) of 80%, forced expiratory volume of 68% (FEV1) in 1 second, and FEV1/FVC of 78%. All other examinations were within normal limits.
No premedication was administrated. Anticholinergic drugs were excluded because they exacerbated the viscosity of the secretion. Vital signs on arrival at the operating room were 117/73 mmHg, 86 beats/min, and SaO2 (oxygen saturation) 97%. Size 2 oropharyngeal airway (Guedel Fix airway, VBM Medizintechnik GmbH, Germany) was selected prior to anesthesia induction and was inserted from outside.
During preoxygenation, anesthesia was induced and maintained with propofol (target blood concentration: 3.0–3.5 μg/ml) and remifentanil (target blood concentration: 2.5–3.0 ng/ml), using a target-controlled infusion device (Orchestra; Fresenius Kabi, Germany). After intravenous injection of 20 mg of rocuronium, the motor response after jaw-thrust was tested to assess adequate anesthetic depth. Tracheal intubation was performed using a single-lumen tube with an internal diameter of 6.5 mm, with a high-volume, low-pressure cuff (Mallinckrodt Medical, Ireland) that was inserted smoothly in a single attempt. Lung sounds were confirmed by stethoscope, and no wheezing or accidental sounds were heard. The patient’s lungs were ventilated with 40% oxygen at a respiration rate of 12 breaths/min and at an inspiratory to expiratory (I : E) ratio of 1 : 2. For mechanical ventilation, a volume-controlled ventilation mode was applied, with a volume of 8–10 ml/kg, with normocarbia throughout the procedure. The anesthetic circuit was electrically humidified (using an A4488 heated and humidified anesthesia breathing circuit, [Mega Acer Kit®, Ace Medical, Korea]). To maintain the bispectral index score between 40 and 60, the concentration of propofol and remifentanil was adjusted. The procedure lasted for 80 min, without using additional muscle relaxant. Throughout the procedure, there were no signs of secretion accumulation. Intraoperatively, an intravenous cefolatam 1,300 mg (cefoperazone sodium 650 mg, sulbactam sodium 650 mg) bolus was administered.
After surgery, administration of all anesthetics was stopped, and the residual neuromuscular block was reversed with 150 mg of sugammadex. Respiratory secretions were aspirated after the operation using endotracheal and oral suction via a catheter inserted as deeply as possible into the trachea. The total amount of yellowish, purulent secretion removed was about 10 cm3 and was hardly sticky. When the patient responded to speech and showed sufficient natural respiration and neuromuscular function, the tube was gently removed. For postoperative analgesia, we administered a 20 μg bolus of intravenous fentanyl. The patient was transferred from the post-anesthetic care room to the general ward 30 min after discontinuation of anesthesia. The patient was closely monitored and antibiotic was administrated by bolus shot. Postoperative chest radiography revealed no active lung lesion (Fig. 1B), possibly due to antibiotic treatment and pre- and postoperative suction therapy. The patient recovered and was discharged on the third day after surgery.
We could obtain approval for publication of this case report from the patient. The pictures of the patient’s face, both pre- and post-surgery, are however not shown, due to the patient’s refusal. All Health Insurance Portability and Accountability Act identifiers have been removed from the report.
We herein represented the anesthetic management of a patient diagnosed with WS during the preoperative workup for surgical treatment of recurrent nasal polyposis with broadening of the nose, frontal sinus aplasia, dyscrinia, and bronchiectasis. The general anesthetic technique used involved target-controlled infusion of propofol and remifentanil.
Management of WS consists of two stages. The first is a complete resection of the nasal polyps, best executed by functional endoscopic sinus surgery (FESS), followed by a second reconstruction stage as septorhinoplasty. FESS is considered the gold standard for the surgical treatment of chronic rhinosinusitis with or without nasal polyps. It could minimize the recurrence rate of the disease. Septorhinoplasty on WS aims to restore nasal function by maximizing nasal airflow and improving cosmetic appearance [9]. Silastic tubes and plates are also left in the nasal cavity to prevent closing of the airspace by fibrous tissue [3].
The main anesthetic consideration when managing patients with WS is the increased risk of pulmonary dysfunction, especially due to intra-operatively acquired respiratory infections, and deterioration of obstructive airway disease [2,4]. The use of disposable airway aids; smooth, non-traumatic airway manipulation, including intubation; and steps to prevent aspiration and irrigation after appropriate neuromuscular recovery is therefore required. Optimal postoperative analgesics are necessary to provide adequate pain relief and avoid excessive sedation [10]. Preoperatively, when there is ciliary dyskinesia, pulmonary status should be managed by chest physiotherapy; infections should be treated, for instance, by antibiotic administration during surgery [5]. As nasal polyps can obstruct the nasal cavity and pharynx, an oropharyngeal airway should be established to assist in preoxygenation before anesthesia induction. Early mobilization and humidification of the inspired gases should assist in the clearance of mucus and may decrease the duration of hospital stay and morbidity [10]. Appropriate pain relief, with early mobilization, postural drainage, and the use of antibiotics, bronchodilators, and oxygenation will decrease the risk of excessive respiratory secretions and intra-operatively acquired infections. The patient should be assisted in removing secretions effectively by oropharyngeal and tracheal suctioning.
Bronchiectasis results in impaired mucociliary clearance, which leads to airway mucus retention and chronic respiratory infection; it can progress to cor pulmonale, pulmonary edema, and amyloidosis, which fortunately did not develop in our patient. Conventional inhaled anesthesia decreases ciliary movement; consequently, perioperative control and precautions against respiratory infections, involving the use of antibiotics and sterile equipment, and prevention of cilio-depressants are also important in anesthetic management [6].
Ledowski et al. [7] found that sevoflurane and remifentanil anesthesia significantly reduced the rate of bronchial mucus transport as compared to propofol and remifentanil. Propofol stimulates ciliary motility in the tracheal epithelium of cultured rats [8]. In addition, propofol facilitates generation of cyclic guanosine monophosphate (cGMP) by the respiratory ciliated epithelium, by stimulating nitric oxide (NO) release from vascular endothelial cells. The NO–cGMP signaling pathway plays an important role in regulating ciliary movement. The bronchial mucus transfer rate in patients having propofol/morphine was significantly higher than those having propofol/remifentanil [11]. Therefore, administration of remifentanil can significantly impair bronchociliary clearance as compared to morphine, which may have clinical implications in patients at particular risk, such as bronchiectasis patients. However, given its ability to regulate respiratory depression, its short context-sensitive half-life, and its superior control of sympathetic nerve stimulation during surgery, we chose to use a low-dose remifentanil infusion. Moreover, ineffective pain control and respiratory depression can lead to chest expansion and ineffective cough, causing basal atelectasis, hypoxemia, and nosocomial infection. Therefore, the selection of appropriate postoperative pain management agents, such as short-acting opioids, is important.
Local anesthesia with sedative may be an alternative for surgery in WS patients, especially in those with respiratory problems. Dogan et al. [12] demonstrated that septoplasty performed under local anesthesia with dexmedetomidine sedation resulted in a more stable hemodynamic state, less surgical bleeding, less nausea vomiting, a shorter recovery period, and less postoperative pain than general anesthesia. This approach has many advantages and disadvantages. There is a risk of aspiration due to intranasal bleeding, and deep suction into the trachea and bronchus. In addition, the intubation and humidification of the breathing gas, cannot be performed without proper coordination which presents the biggest limitation of local anesthesia. Local anesthesia was excluded in the patient presented here due to his age and the drawbacks of this approach. Additionally, in a meta-analysis, Al-Moraissi and Ellis [13] showed that general anesthesia tends to yield better outcomes in terms of satisfaction with anesthesia, nasal function, and subsequent treatments (septoplasty, septorhinoplasty, rhinoplasty, and refracture).
The extreme broadening of our patient’s nose may have been due to the ongoing pressure exerted by polyps, in accordance with the hypertrophic process and nasal pyramid deformation. The chronic pressure also leads to an enlargement of the lamina papyracea, with progressive bulbous protrusion laterally, and increases the risk of slowly progressing blindness due to compression of the optic nerve. Abbud-Neme et al. [3] reported rhinoplasty as a part of the treatment for WS; however, treatment is more typically limited to ESS. Schoenenberger and Tasman [14] described that nasal bone atrophy in WS was sufficiently severe to allow fracturing without osteotomy, as the bone was thinned to the extent of giving way merely upon compression with a digit. This allowed straight forward, efficient adjustment of the nasal form, which the patient considered very satisfactory. The nose enlargement due to the abnormal growth implies a risk of breakage and optic nerve damage during mask fitting or palpation, which is dangerous, because the ensuing bleeding can cause aspiration, and hence, appropriate care should be taken while handling the nose during induction. Likewise, in our case, by reducing the pressure slightly, the air pressure in the mask was reduced, and thereby we prevented leakage of air between the mask and the nose.
Fortunately, this is a rare disease. When a child with nasal polyps and broadening of the nose is encountered, bronchiectasis must be ruled out; if this is done, a diagnosis of WS may be plausible. Nasal resection and ESS should be performed early to avoid excessive facial deformation.
In summary, we here described a case of successful anesthetic management of both ESS and septorhinoplasty in a 13-year-old patient with WS. We presented a suitable anesthetic management approach using total intravenous anesthesia with propofol and remifentanil, and delineated precautions that should be taken for patients with WS. We also discussed the anesthetic implications of bronchiectasis and ciliary motility impairment. Following these recommendations should allow uncomplicated anesthetic management of patients with WS.
References
1. Woakes E. Necrotising ethmoiditis and mucous polyps. Lancet. 1885; 61:619–20.
2. Kellerhals B, de Uthemann B. Woakes' syndrome: the problems of infantile nasal polyps. Int J Pediatr Otorhinolaryngol. 1979; 1:79–85.

3. Abbud-Neme F, Reynoso VM, Deutsch Reiss E. Woake's syndrome. A case report in a teenager. Int J Pediatr Otorhinolaryngol. 1987; 12:327–33.

4. Groman JD, Bolger W, Brass-Ernst L, Macek M Jr, Zeitlin P, Cutting G. Recurrent and destructive nasal polyposis in 2 siblings: a possible case of Woakes' syndrome. Otolaryngol Head Neck Surg. 2004; 131:1009–11.

5. Jo YY, Jung WS, Kim HS, Byen SH, Lee KC. Total intravenous anesthesia for Kartagener's syndrome: a case report. Anesth Pain Med. 2012; 7:317–9.
6. Raphael JH, Strupish J, Selwyn DA, Hann HC, Langton JA. Recovery of respiratory ciliary function after depression by inhalation anaesthetic agents: an in vitro study using nasal turbinate explants. Br J Anaesth. 1996; 76:854–9.

7. Ledowski T, Paech MJ, Patel B, Schug SA. Bronchial mucus transport velocity in patients receiving propofol and remifentanil versus sevoflurane and remifentanil anesthesia. Anesth Analg. 2006; 102:1427–30.

8. Shirakami G, Li D, Zhan X, Johns RA. Propofol stimulates ciliary motility via the nitric oxide-cyclic GMP pathway in cultured rat tracheal epithelial cells. Anesthesiology. 2000; 93:482–8.

9. Chisholm E, Jallali N. Rhinoplasty and septorhinoplasty outcome evaluation. Ear Nose Throat J. 2012; 91:10–4.

10. Larrow V, Klich-Heartt EI. Prevention of ventilator-associated pneumonia in the intensive care unit: beyond the basics. J Neurosci Nurs. 2016; 48:160–5.
11. Ledowski T, Hilmi S, Paech MJ. Bronchial mucus transport velocity in patients receiving anaesthesia with propofol and morphine or propofol and remifentanil. Anaesthesia. 2006; 61:747–51.

12. Dogan R, Erbek S, Gonencer HH, Erbek HS, Isbilen C, Arslan G. Comparison of local anaesthesia with dexmedetomidine sedation and general anaesthesia during septoplasty. Eur J Anaesthesiol. 2010; 27:960–4.

13. Al-Moraissi EA, Ellis E 3rd. Local versus general anesthesia for the management of nasal bone fractures: a systematic review and meta-analysis. J Oral Maxillofac Surg. 2015; 73:606–15.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download