Abstract
Background
Duchenne muscular dystrophy (DMD) is the most common childhood muscular dystrophy that anesthesiologists can encounter in the operation room, and patients with DMD are susceptible to complications such as rhabdomyolysis, hyperkalemic cardiac arrest, and hyperthermia during the perioperative period. Acute onset of hyperkalemic cardiac arrest is a crisis because of the difficulty in achieving satisfactory resuscitation owing to the sustained hyperkalemia accompanied by rhabdomyolysis.
Case
We here report a case of a 13-year-old boy who had multiple leg fractures and other trauma after a car accident and who had suffered from acute hyperkalemic cardiac arrest. He was refractory to cardiopulmonary resuscitation and showed sustained hyperkalemia. With extracorporeal membrane oxygenation and in-line hemofiltration, he recovered from repeated cardiac arrest and hyperkalemia.
For anesthesiologists, Duchenne muscular dystrophy (DMD) is the most common childhood muscular dystrophy, with an incidence of three cases per 100,000 of the population [1]. Various anesthesia-related perioperative risks such as rhabdomyolysis, hyperkalemic cardiac arrest, and hyperthermia exist in DMD patients. Complications in DMD occur most frequently after orthopedic surgery [2], and one of the important clinical presentations of DMD during anesthesia is acute onset of hyperkalemic cardiac arrest with no obvious preceding signs of hypermetabolism [3].
In cases of severe rhabdomyolysis and hyperkalemia in myopathy patients, it is difficult to achieve satisfactory resuscitation and successful return of spontaneous circulation and additional acute kidney injury (AKI) is considered. Thus, extracorporeal membrane oxygenation (ECMO) support and hemodialysis support can be helpful for these patients [4].
We here report a case of a successful application of a combination of ECMO and in-line hemofiltration for a patient who suffered acute hyperkalemic cardiac arrest after orthopedic surgery. He was refractory to cardiopulmonary resuscitation (CPR) and showed sustained hyperkalemia. After the use of ECMO and in-line hemofiltration, he recovered from the repeated cardiac arrest and hyperkalemia.
A 13-year-old boy (height: 163 kg; weight: 54 kg) was admitted because of fractures of the left femur and ulna and the nasal bone as well as a blowout fracture after a car accident; he also had contusions to the chest wall, heart, and liver. According to his medical history, he had been diagnosed with DMD when he was three years old and had taken steroids. He had mild weakness in his lower limbs, but he could walk normally, and he did not complain of any respiratory distress. We referred the patient to the pediatric department for a preoperative evaluation, and he had no cardiac complications; his electrocardiogram (ECG) also showed normal sinus rhythm with right ventricular hypertrophy. Most of the laboratory findings were normal, but his abnormal findings were as follows: aspartate transaminase, 156.0 U/L; alanine transaminase, 215.7 U/L; creatine kinase (CK), 11196 U/L; CK-MB, 132.6 ng/ml. Mild rhabdomyolysis due to trauma was suspected, and the patient received hydration therapy. His K+ level was 4.00 mEq/L, and his urine output was more than 800 ml/day before surgery. The orthopedic surgeon planned to delay the operation until the swelling subsided and laboratory findings improved; however, he decided to conduct emergency surgery only for the femur fracture because of the risk of nonunion.
Three days after the accident, the patient was transferred to the operating room (OR) for the emergent surgery; baseline measurements were oxygen saturation (SpO2) 100%, noninvasive blood pressure 120/80 mmHg, heart rate (HR) 120 beats/min, end-tidal CO2 (ETCO2) 39 mmHg, and esophageal temperature 37.4oC. Anesthesia was induced using 250 mg of thiopental sodium and 35 mg of rocuronium and was maintained using 1% vol of sevoflurane with a fresh gas flow of 3 L/min of N2O/oxygen mixture. At the end of surgery, he recovered adequate respiration (train-of-four > 99%) with 10 mg of pyridostigmine and 0.4 mg of glycopyrrolate. The surgery time was 2 hours, and the administered fluid and estimated blood loss were 600 ml and 20 ml, respectively. All of his vital signs were maintained within 20% of the baseline, and there were no changes on the ECG or specific complications during the surgery. The patient’s mental state was clear, and he was transferred to the recovery room.
On arrival in the recovery room, the patient could answer questions, and he complained of pain in his leg. His vital signs were noninvasive blood pressure 119/70 mmHg, HR 90 beats/min, SpO2 98%, and ear temperature 36.3oC (Table 1). Approximately 2 minutes later, he became cyanotic and apneic, and his HR on the ECG showed 7 beats/min; the ECG showed an irregular ventricular wide-complex sine wave without a p wave. CPR was started, and 1 mg of epinephrine was administered. During the CPR, the patient underwent endotracheal intubation and arterial blood gas analysis (ABGA), and the ABGA was repeated approximately every 15 minutes after the CPR. An arterial blood gas test revealed pH 6.530, PaO2 38.9 mmHg, PaCO2 97.8 mmHg, base excess (BE) −32.4 mEq/L, Na+ 132.8 mEq/L, K+ 9.60 mEq/L, and hematocrit 38% (Table 1). Cardiac arrest due to hyperkalemia was suspected, and 20 ml of 3% calcium chloride, 10 unit of regular insulin, and 80 mEq of sodium bicarbonate were administered (Fig. 1); the patient achieved return of spontaneous circulation, and his blood pressure and heart rate became 80/35 mmHg and 40 beats/min, respectively. He was moved back to the OR for further monitoring and management and for effective CPR. External pacing was attempted, but there was no response. Continuous infusions of dobutamine, norepinephrine, and vasopressin were started, and mechanical ventilation (MV) was maintained with 5 L/min of 100% O2. However, cardiovascular collapse developed again about 5 minutes later and CPR was applied. Despite treatment with multiple doses of insulin, calcium chloride, and sodium bicarbonate, the patient’s K+ concentration did not reduce below 8.06 mmol/Eq, and the cardiovascular collapse repeated itself. Moreover, pulmonary edema developed, and peak inspiratory pressure increased more than 40 mmHg. The ABGA showed sustained acidosis (pH < 7.0), hypercarbia (PaCO2 > 90 mmHg), hypoxia (PaO2 < 50 mmHg), and hyperkalemia (K+ > 8.06 mEq/L).
After the fourth cardiovascular collapse, we decided to apply ECMO. We consulted with the cardiothoracic surgeon about using ECMO not only for cardiac and respiratory support but also to eliminate excess K+ using hemofiltration. The cardiothoracic surgeon suggested the use of in-line hemofiltration with ECMO and prepared for surgery; arterial and venous cannulas (Biomedicus®, Medtronic, USA) were inserted into the patient’s right femoral artery and vein. The hemofilter (Hemofiltration kit, Dideco, Italy) was connected between the pump and the arterial line of the ECMO circuit to remove excess K+ and water. ECMO was started 90 minutes after the first cardiac arrest, and blood flow was maintained about 3.0 L/min.
About 30 minutes after starting ECMO, laboratory results showed significant improvement and there was no further cardiac arrest (Table 1 and Fig. 1). About 1 hour after the ECMO, ABGA showed almost normal pH (7.289), PaCO2 (38.9 mmHg), and PaO2 (200.8 mmHg), and lower serum K+ concentration (5.97 mEq/L). During the CPR and surgery for ECMO, 3,000 ml of fluids were administered, urine output was 300 ml, and estimated volume of bleeding was 200 ml. Then, the patient was transferred to the intensive care unit (ICU) and sedated using dexmedetomidine and remifentanil. Under suspicion of severe rhabdomyolysis, the patient’s serum CK, myoglobin, and urine myoglobin were measured. CK had increased to 242,340 U/L after cardiac arrest and decreased to 539 U/L after 26 days of treatment (Table 2), and serum and urine myoglobin had increased by more than 3,000 and 20,000 ng/ml, respectively, both of which exceeded the measurable value, and decreased to 2,359 and 1,438 ng/ml after 20 days, also respectively (Table 2).
In the ICU, the patient remained on ECMO support for 3 days. Although he had suffered from pneumonia, his vital signs were improving, but AKI secondary to myoglobinemia developed. At first, the patient maintained urine output more than 500 ml/h, but urine output decreased to less than 80 ml/h on the 3rd day after the ECMO. Moreover, intra-arterial thrombosis of the right femoral artery developed. Thus, we weaned the patient from the ECMO and MV, performed angioplasty of the femoral artery, and applied continuous renal replacement therapy (CRRT) simultaneously because his vital signs stabilized. The patient was successfully weaned from ECMO and MV, and CRRT was started with a newly inserted central venous catheter in the left internal jugular vein. His condition improved daily, and all inotropics and vasopressors were stopped on the 5th day. After 10 days from the start of CRRT, the patient’s renal functions had recovered, and he was successfully weaned from CRRT.
The patient was discharged from the ICU after 21 days of treatment and moved to the ward. Unfortunately, he required surgical disarticulation of his right leg because of angiopathy following the ECMO and the long rehabilitation treatment. However, he successfully recovered from the cardiac arrest by hyperkalemia due to massive rhabdomyolysis with the combination of ECMO and in-line hemofiltration.
In this case report, a boy with DMD developed repeated hyperkalemic cardiac arrest that was refractory to CPR but successfully recovered with the combination of ECMO and in-line hemofiltration.
DMD is a rare disease characterized by the total absence of dystrophin caused by recessive mutations in the dystrophin gene [2]; dystrophin protects sarcolemmas from contractile stresses by mechanical reinforcement. Skeletal muscle tissues in patients with DMD can be destroyed after exposure to triggers and can lead to rhabdomyolysis; one of the known triggers is succinylcholine, which we therefore avoided. Volatile anesthetics are thought to be associated with rhabdomyolysis in patients with DMD, but most of the cases developed after use of sevoflurane with succinylcholine [2]. In addition, there remains the controversy regarding the relationship with rhabdomyolysis [2,5], and total intravenous anesthesia rather than volatile anesthetics was reiterated in a nonevidence-based recommendation by the American College of Chest Physicians [6] because there is no ‘risk-free’ drug [5]. Thus, we believed that there was little evidence that sevoflurane is directly involved in the complications of patients with DMD, and we opted to use sevoflurane to maintain anesthesia. Moreover, the patient in our case report was over eight years old, which we believed made him less susceptible to rhabdomyolysis after exposure to anesthetics because of the limited number or absence of unstable muscle fibers [2]. During the surgery, there were no signs of hyperkalemia or rhabdomyolysis, but hyperkalemic cardiac arrest with rhabdomyolysis developed after the patient was transferred to the recovery room. We believed that possible causes of the rhabdomyolysis were immobilization after the trauma, the surgery itself, or excessive muscle activity during emergence [2,7,8]. The patient had been immobilized for three days after a car accident and underwent orthopedic surgery, during which the majority of perioperative complications occur in patients with DMD [2]. In this case report, we reported on a pediatric patient who had more prominent muscle activity as he emerged from anesthesia, and excessive muscle activity during emergence from anesthesia may play an important role in rhabdomyolysis [7]. However, the exact cause of the delayed onset of hyperkalemic cardiac arrest with rhabdomyolysis is unclear.
Since ECMO was introduced in extracorporeal technology and in clinical medicine, clinical practice has made remarkable advances [9]. In particular, ECMO can be a therapeutic option for managing refractory cardiorespiratory failure [9]. Cardiac arrest could be refractory to CPR and prolonged in acute hyperkalemic cardiac arrest with rhabdomyolysis in patients with myopathy because of sustained hyperkalemia [10]. Previous reports showed that cardiopulmonary bypass and ECMO could be therapeutic options for cardiopulmonary support for refractory cardiac arrest associated with hyperkalemia or rhabdomyolysis [4,10,11]. In particular, ECMO can be recommended for prolonged refractory cardiac arrest due to reversible etiology because it has shown acceptable survival rates with minimal neurologic damage [4,12]. Fortunately, the patient in this case report also survived without neurologic deficit despite serial cardiac arrest that occurred four times over one hour. We believed that the patient survived for a number of reasons: the cardiac arrest was noticed quickly and the patient received CPR immediately, the patient received highly effective CPR in the OR, the main cause of the cardiac arrest was hyperkalemia—which was reversible—and the hyperkalemia was controlled by early application of ECMO with in-line hemofiltration and CRRT.
Most of all, we believed that the simultaneous application of in-line hemofiltration with ECMO was effective for preventing repeated cardiac arrest due to hyperkalemia. In patients with AKI, fluid overload and metabolic disturbances such as hyperkalemia are common; the incidence of AKI is 70%–85% in ECMO patients [13,14]. In these patients, CRRT on ECMO can be performed by using in-line hemofiltration or incorporating a standard CRRT machine into the ECMO circuit. The patient in the current case suffered from repeated cardiac arrest, hyperkalemia, and also suspected pulmonary edema due to fluid administration during CPR. Thus, adding in-line hemofiltration in the ECMO was very effective in decreasing his fluid overload and correcting his hyperkalemia [14]. The patient’s ongoing hyperkalemia decreased below 6.00 mEq/L and his PaO2 increased to more than 100 mmHg after we started the ECMO. As we expected, the patient developed AKI due to increased rhabdomyolysis, and in-line hemofiltration effectively controlled his volume and electrolyte status. When the patient’s vital signs became stable, in-line hemofiltration was replaced with conventional CRRT after we removed the ECMO.
We used in-line hemofiltration as we implanted the ECMO; however, there are some disadvantages in this method compared with just including CRRT in the ECMO circuit. Because the fibers of in-line hemofilters are designed for use with high-pressure systems, diffusive clearance is less effective than that with conventional CRRT [13], and filter clotting or rupture cannot be detected early because the blood flow to the hemofilter cannot be controlled or monitored, and these limitations make in-line hemofiltration less accurate and effective [13,14]; thus, including a CRRT in the ECMO circuit is a safer and more effective technique with fewer complications. However, in-line hemofiltration is relatively simple and inexpensive and can be applied quickly [14]. We used in-line hemofiltration to reduce serum K+ level concurrently with ECMO for the cardiac arrest, and the patient could have recovered from the hyperkalemia quickly. With these advantages, we suggest that in-line hemofiltration can be applied more easily during emergency situations such as refractory hyperkalemic cardiac arrest.
In summary, we present the case of a 13-year-old male with DMD who developed repeated hyperkalemic cardiac arrest with rhabdomyolysis following orthopedic surgery. The surgeon used ECMO with in-line hemofiltration because the patient was refractory to cardiopulmonary resuscitation due to ongoing hyperkalemia, but he recovered without neurological damage. Combining ECMO and in-line hemofiltration might be a safe and effective technique for refractory hyperkalemic cardiac arrest and rhabdomyolysis in patients with DMD.
References
1. Morris P. Duchenne muscular dystrophy: a challenge for the anaesthetist. Paediatr Anaesth. 1997; 7:1–4.

2. Segura LG, Lorenz JD, Weingarten TN, Scavonetto F, Bojanić K, Selcen D, et al. Anesthesia and Duchenne or Becker muscular dystrophy: review of 117 anesthetic exposures. Paediatr Anaesth. 2013; 23:855–64.

3. Hayes J, Veyckemans F, Bissonnette B. Duchenne muscular dystrophy: an old anesthesia problem revisited. Paediatr Anaesth. 2008; 18:100–6.

4. Al-Takrouri H, Martin TW, Mayhew JF. Hyperkalemic cardiac arrest following succinylcholine administration: The use of extracorporeal membrane oxygenation in an emergency situation. J Clin Anesth. 2004; 16:449–51.

5. Hopkins PM. Anaesthesia and the sex-linked dystrophies: between a rock and a hard place. Br J Anaesth. 2010; 104:397–400.

6. Birnkrant DJ. The American College of Chest Physicians consensus statement on the respiratory and related management of patients with Duchenne muscular dystrophy undergoing anesthesia or sedation. Pediatrics. 2009; 123 Suppl 4:S242–4.

7. Gaschen F, Gaschen L, Seiler G, Welle M, Jaunin VB, Jmaa DG, et al. Lethal peracute rhabdomyolysis associated with stress and general anesthesia in three dystrophin-deficient cats. Vet Pathol. 1998; 35:117–23.

8. Zutt R, van der Kooi AJ, Linthorst GE, Wanders RJ, de Visser M. Rhabdomyolysis: review of the literature. Neuromuscul Disord. 2014; 24:651–9.

9. MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med. 2012; 38:210–20.

10. Lee G, Antognini JF, Gronert GA. Complete recovery after prolonged resuscitation and cardiopulmonary bypass for hyperkalemic cardiac arrest. Anesth Analg. 1994; 79:172–4.

11. Huh H, Jung JS, Park SJ, Park MK, Lim CH, Yoon SZ. Successful early application of extracorporeal membrane oxygenation to support cardiopulmonary resuscitation for a patient suffering from severe malignant hyperthermia and cardiac arrest: a case report. Korean J Anesthesiol. 2017; 70:345–9.

12. Lee JH, Park SH, Song IA. Cardiopulmonary resuscitation with assisted extracorporeal life support during cardiac arrest caused by drugeluting stent thrombosis: a case report. Korean J Anesthesiol. 2014; 66:383–7.

Fig. 1.
Timeline of cardiopulmonary resuscitation and patient treatment. CPR: cardiopulmonary resuscitation, ROSC: return of spontaneous circulation, ABGA: arterial blood gas analysis, ECMO: extracorporeal membrane oxygenation, MV: mechanical ventilation, CRRT: continuous renal replacement therapy.
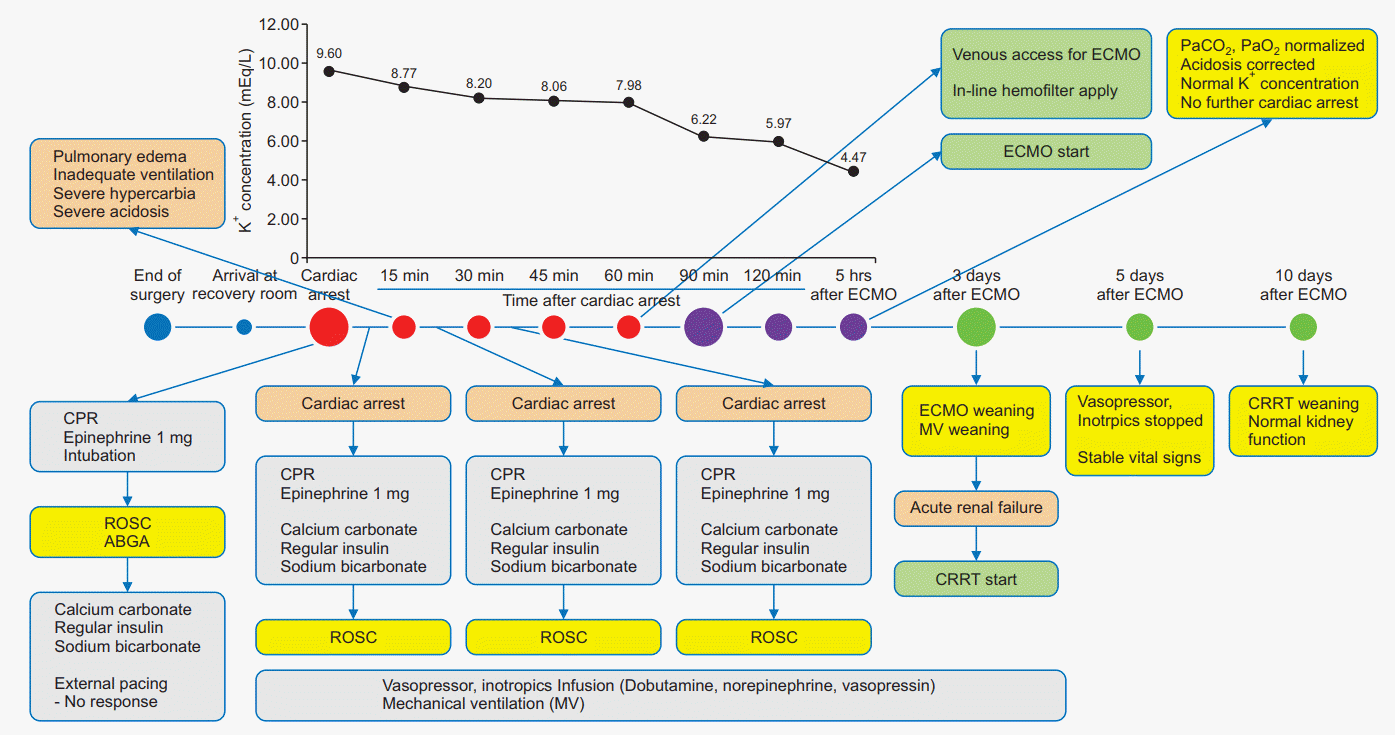
Table 1.
Changes in Vital Signs, ABGA, Electrolytes, and Glucose during CPR and ECMO
|
At recovery room |
After cardiac arrest |
After ECMO |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arrival or Preoperation | Cardiac arrest | 15 min | 30 min | 45 min | 60 min | 90 min* | 120 min | 5 h | 1 day | 3 days | ||
| Vital signs | BP (mmHg) | 119/70 | 65/9 | 80/35 | 70/35 | 110/50 | 55/30 | 58/30 | 68/30 | 70/40 | 100/50 | 120/60 |
| HR (beats/min) | 90 | 7 | 40 | 45 | 105 | 120 | 98 | 90 | 108 | 115 | 145 | |
| SpO2 (%) | 98 | 70 | 96 | 98 | 97 | 98 | ||||||
| BT (oC) | 36.3 | 35.3 | 35.0 | 34.8 | 34.8 | 35.4 | 35.5 | 35.0 | 35.7 | 35.9 | ||
| BIS | 50 | 52 | 30 | 28 | 47 | 15 | ||||||
| ETCO2 (mmHg) | 18 | 30 | 48 | 33 | 14 | 17 | ||||||
| ABGA | Hb (g/dl) | 13.2 | 12.8 | 10.7 | 9.4 | 9.9 | 7.9 | 9.6 | 9.3 | 10.5 | ||
| pH | 6.530 | 6.695 | 7.029 | 6.776 | 6.887 | 6.939 | 7.289 | 7.258 | ||||
| PaCO2 (mmHg) | 97.8 | 123.3 | 131.5 | 101.7 | 97.3 | 44.5 | 38.9 | 27.6 | ||||
| PaO2 (mmHg) | 38.9 | 46.7 | 43.0 | 46.6 | 45.3 | 114.3 | 200.8 | 447 | ||||
| HCO3− (mmol/L) | 2.8 | 7.4 | 24.6 | 8.5 | 8.3 | 8.1 | 18.9 | 12.5 | ||||
| BE (mmol/L) | −30.9 | −21.5 | 3.1 | −20.3 | −22.5 | −22.9 | −6.9 | −12.6 | ||||
| Na+ (mEq/L) | 133.0 | 132.8 | 137.2 | 150.4 | 140.9 | 141.9 | 143.3 | 146.9 | 141.5 | |||
| K+ (mEq/L) | 4.00 | 9.60 | 8.77 | 8.20 | 8.06 | 7.98 | 6.22 | 5.97 | 4.47 | |||
| Ca++ (mEq/L) | 1.04 | 0.77 | 0.72 | 0.92 | 0.86 | 0.75 | 0.98 | |||||
| Glucose (mg/dl) | 122 | 405 | 324 | 272 | 276 | 208 | 202 | |||||
Table 2.
Changes in Chemistry before and after Cardiac Arrest and ECMO




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download