2. Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000; 39:215–31.
3. Pai MP, Paloucek FP. The origin of the “ideal” body weight equations. Ann Pharmacother. 2000; 34:1066–9.

4. James WP. Research on obesity. London: Her Majesty’s Stationery Office;1976.
5. Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005; 44:1051–65.

6. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987; 317:1098.
7. Shibutani K, Inchiosa MA Jr, Sawada K, Bairamian M. Accuracy of pharmacokinetic models for predicting plasma fentanyl concentrations in lean and obese surgical patients: derivation of dosing weight (“pharmacokinetic mass”). Anesthesiology. 2004; 101:603–13.
8. Abernethy DR, Burckart GJ. Pediatric dose selection. Clin Pharmacol Ther. 2010; 87:270–1.

9. Mahmood I. Prediction of clearance, volume of distribution and half-life by allometric scaling and by use of plasma concentrations predicted from pharmacokinetic constants: a comparative study. J Pharm Pharmacol. 1999; 51:905–10.

10. Mahmood I. Prediction of drug clearance in children: impact of allometric exponents, body weight, and age. Ther Drug Monit. 2007; 29:271–8.

11. Adams JP, Murphy PG. Obesity in anaesthesia and intensive care. Br J Anaesth. 2000; 85:91–108.

12. Shenkman Z, Shir Y, Brodsky JB. Perioperative management of the obese patient. Br J Anaesth. 1993; 70:349–59.

13. Casati A, Putzu M. Anesthesia in the obese patient: pharmacokinetic considerations. J Clin Anesth. 2005; 17:134–45.

14. Kendrick JG, Carr RR, Ensom MH. Pharmacokinetics and drug dosing in obese children. J Pediatr Pharmacol Ther. 2010; 15:94–109.

15. Shi S, Klotz U. Age-related changes in pharmacokinetics. Curr Drug Metab. 2011; 12:601–10.

16. Jung D, Mayersohn M, Perrier D, Calkins J, Saunders R. Thiopental disposition in lean and obese patients undergoing surgery. Anesthesiology. 1982; 56:269–74.

17. Servin F, Farinotti R, Haberer JP, Desmonts JM. Propofol infusion for maintenance of anesthesia in morbidly obese patients receiving nitrous oxide. A clinical and pharmacokinetic study. Anesthesiology. 1993; 78:657–65.

18. Lesser GT, Deutsch S. Measurement of adipose tissue blood flow and perfusion in man by uptake of 85Kr. J Appl Physiol. 1967; 23:621–30.

19. Björkman S, Wada DR, Stanski DR. Application of physiologic models to predict the influence of changes in body composition and blood flows on the pharmacokinetics of fentanyl and alfentanil in patients. Anesthesiology. 1998; 88:657–67.
20. Avram MJ, Krejcie TC. Using front-end kinetics to optimize target-controlled drug infusions. Anesthesiology. 2003; 99:1078–86.

21. Collis T, Devereux RB, Roman MJ, de Simone G, Yeh J, Howard BV, et al. Relations of stroke volume and cardiac output to body composition: the strong heart study. Circulation. 2001; 103:820–5.
22. Abernethy DR, Greenblatt DJ, Divoll M, Smith RB, Shader RI. The influence of obesity on the pharmacokinetics of oral alprazolam and triazolam. Clin Pharmacokinet. 1984; 9:177–83.

23. Salazar DE, Corcoran GB. Predicting creatinine clearance and renal drug clearance in obese patients from estimated fat-free body mass. Am J Med. 1988; 84:1053–60.

24. Stokholm KH, Brøchner-Mortensen J, Hoilund-Carlsen PF. Increased glomerular filtration rate and adrenocortical function in obese women. Int J Obes. 1980; 4:57–63.
25. Benedek IH, Fiske WD 3rd, Griffen WO, Bell RM, Blouin RA, McNamara PJ. Serum alpha 1-acid glycoprotein and the binding of drugs in obesity. Br J Clin Pharmacol. 1983; 16:751–4.

26. Barbeau P, Litaker MS, Woods KF, Lemmon CR, Humphries MC, Owens S, et al. Hemostatic and inflammatory markers in obese youths: effects of exercise and adiposity. J Pediatr. 2002; 141:415–20.

27. Derry CL, Kroboth PD, Pittenger AL, Kroboth FJ, Corey SE, Smith RB. Pharmacokinetics and pharmacodynamics of triazolam after two intermittent doses in obese and normal-weight men. J Clin Psychopharmacol. 1995; 15:197–205.

28. Wasan KM, Lopez-Berestein G. The influence of serum lipoproteins on the pharmacokinetics and pharmacodynamics of lipophilic drugs and drug carriers. Arch Med Res. 1993; 24:395–401.
29. Lloret Linares C, Declèves X, Oppert JM, Basdevant A, Clement K, Bardin C, et al. Pharmacology of morphine in obese patients: clinical implications. Clin Pharmacokinet. 2009; 48:635–51.
30. Ramzan I, Wong BK, Corcoran GB. Pain sensitivity in dietary-induced obese rats. Physiol Behav. 1993; 54:433–5.

31. Maffiuletti NA, Morelli A, Martin A, Duclay J, Billot M, Jubeau M, et al. Effect of gender and obesity on electrical current thresholds. Muscle Nerve. 2011; 44:202–7.

32. McVinnie DS. Obesity and pain. Br J Pain. 2013; 7:163–70.

33. Majchrzak M, Brzecka A, Daroszewski C, Blasiak P, Rzechonek A, Tarasov VV, et al. Increased pain sensitivity in obese patients after lung cancer surgery. Front Pharmacol. 2019; 10:626.

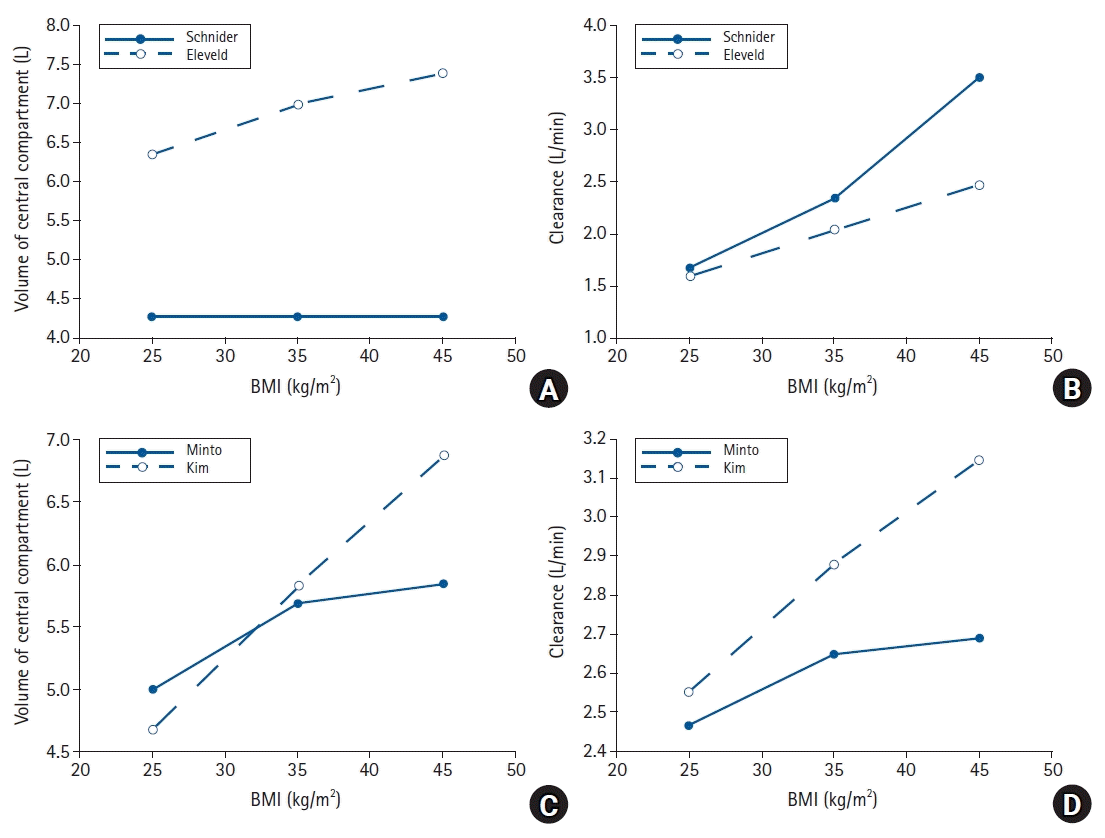
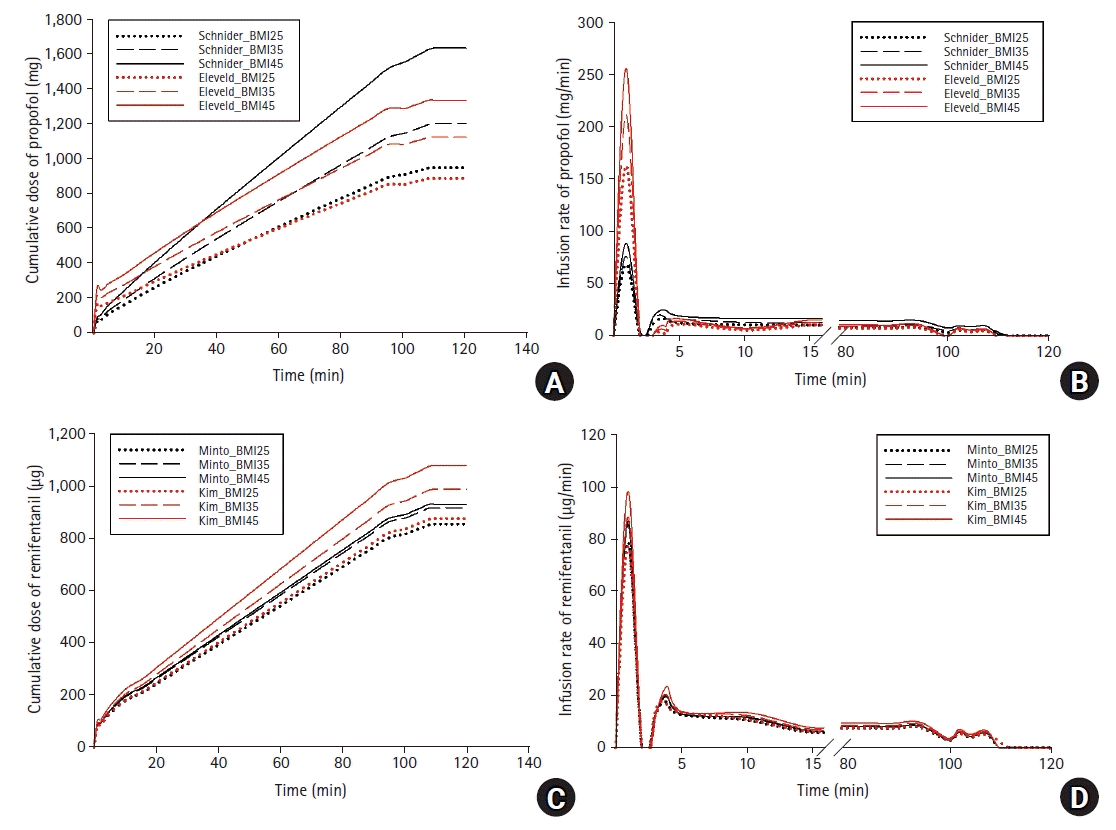
34. Cortínez LI, Anderson BJ, Penna A, Olivares L, Muñoz HR, Holford NH, et al. Influence of obesity on propofol pharmacokinetics: derivation of a pharmacokinetic model. Br J Anaesth. 2010; 105:448–56.
35. Dong D, Peng X, Liu J, Qian H, Li J, Wu B. Morbid obesity alters both pharmacokinetics and pharmacodynamics of propofol: dosing recommendation for anesthesia induction. Drug Metab Dispos. 2016; 44:1579–83.

36. Subramani Y, Riad W, Chung F, Wong J. Optimal propofol induction dose in morbidly obese patients: a randomized controlled trial comparing the bispectral index and lean body weight scalar. Can J Anaesth. 2017; 64:471–9.

37. Cortínez LI, Anderson BJ. Advances in pharmacokinetic modeling: target controlled infusions in the obese. Curr Opin Anaesthesiol. 2018; 31:415–22.
38. Eleveld DJ, Proost JH, Cortínez LI, Absalom AR, Struys MM. A general purpose pharmacokinetic model for propofol. Anesth Analg. 2014; 118:1221–37.

39. Vereecke HE, Eleveld DJ, Colin P, Struys MM. Performance of the eleveld pharmacokinetic model to titrate propofol in an obese Japanese patient population. Eur J Anaesthesiol. 2016; 33:58–9.

40. Aldegheri G, Di Candia D, Gigli F. Predictive performance of the 'Minto' remifentanil pharmacokinetic parameter set in morbidly obese patients ensuing from a new method for calculating lean body mass. Clin Pharmacokinet. 2010; 49:131–9.
41. La Colla L, Albertin A, La Colla G. Pharmacokinetic model-driven remifentanil administration in the morbidly obese: the ‘critical weight’ and the ‘fictitious height’, a possible solution to an unsolved problem? Clin Pharmacokinet. 2009; 48:397–8.
42. Kim TK, Obara S, Egan TD, Minto CF, La Colla L, Drover DR, et al. Disposition of remifentanil in obesity: a new pharmacokinetic model incorporating the influence of body mass. Anesthesiology. 2017; 126:1019–32.
43. Cortínez LI, De la Fuente N, Eleveld DJ, Oliveros A, Crovari F, Sepulveda P, et al. Performance of propofol target-controlled infusion models in the obese: pharmacokinetic and pharmacodynamic analysis. Anesth Analg. 2014; 119:302–10.
44. Eleveld DJ, Colin P, Absalom AR, Struys MM. Pharmacokinetic-pharmacodynamic model for propofol for broad application in anaesthesia and sedation. Br J Anaesth. 2018; 120:942–59.

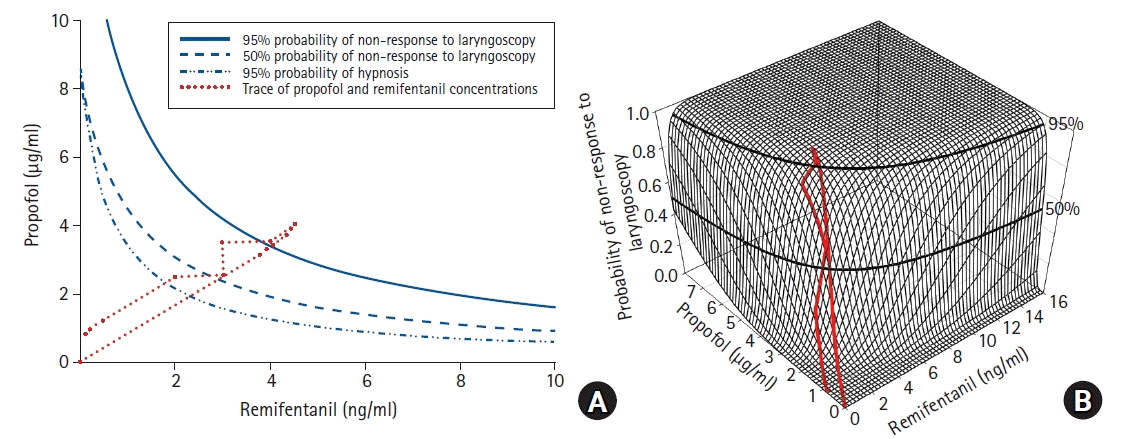
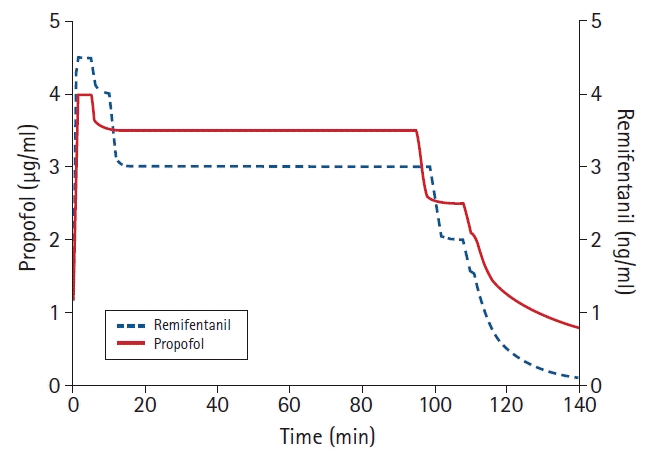
45. Bouillon TW, Bruhn J, Radulescu L, Andresen C, Shafer TJ, Cohane C, et al. Pharmacodynamic interaction between propofol and remifentanil regarding hypnosis, tolerance of laryngoscopy, bispectral index, and electroencephalographic approximate entropy. Anesthesiology. 2004; 100:1353–72.

46. Yufune S, Takamatsu I, Masui K, Kazama T. Effect of remifentanil on plasma propofol concentration and bispectral index during propofol anaesthesia. Br J Anaesth. 2011; 106:208–14.

47. Yi JM, Doh I, Lee SH, Kim SY, Lee YH, Lee EK, et al. Predictive performance of a new pharmacokinetic model for propofol in underweight patients during target-controlled infusion. Acta Anaesthesiol Scand. 2019; 63:448–54.

48. Gutt CN, Oniu T, Mehrabi A, Schemmer P, Kashfi A, Kraus T, et al. Circulatory and respiratory complications of carbon dioxide insufflation. Dig Surg. 2004; 21:95–105.

49. Nguyen NT, Ho HS, Fleming NW, Moore P, Lee SJ, Goldman CD, et al. Cardiac function during laparoscopic vs open gastric bypass. Surg Endosc. 2002; 16:78–83.

50. Perilli V, Sollazzi L, Modesti C, Annetta MG, Sacco T, Bocci MG, et al. Comparison of positive end-expiratory pressure with reverse Trendelenburg position in morbidly obese patients undergoing bariatric surgery: effects on hemodynamics and pulmonary gas exchange. Obes Surg. 2003; 13:605–9.

51. Shibutani K, Inchiosa MA Jr, Sawada K, Bairamian M. Pharmacokinetic mass of fentanyl for postoperative analgesia in lean and obese patients. Br J Anaesth. 2005; 95:377–83.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download