Abstract
References
Fig. 1.
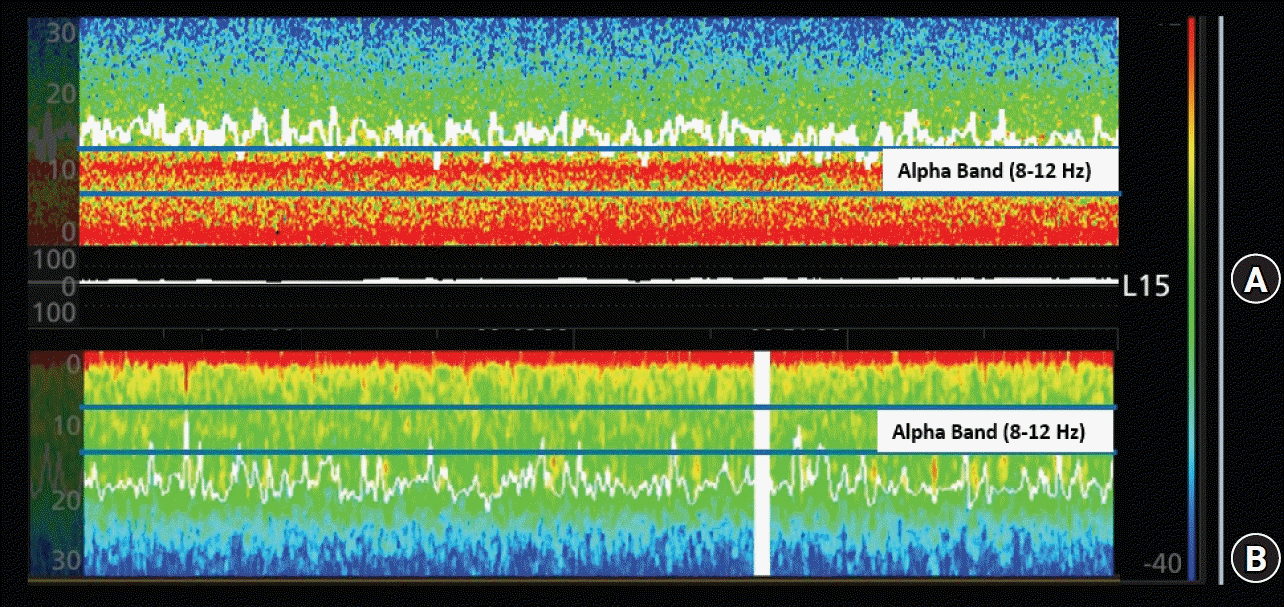
Fig. 2.
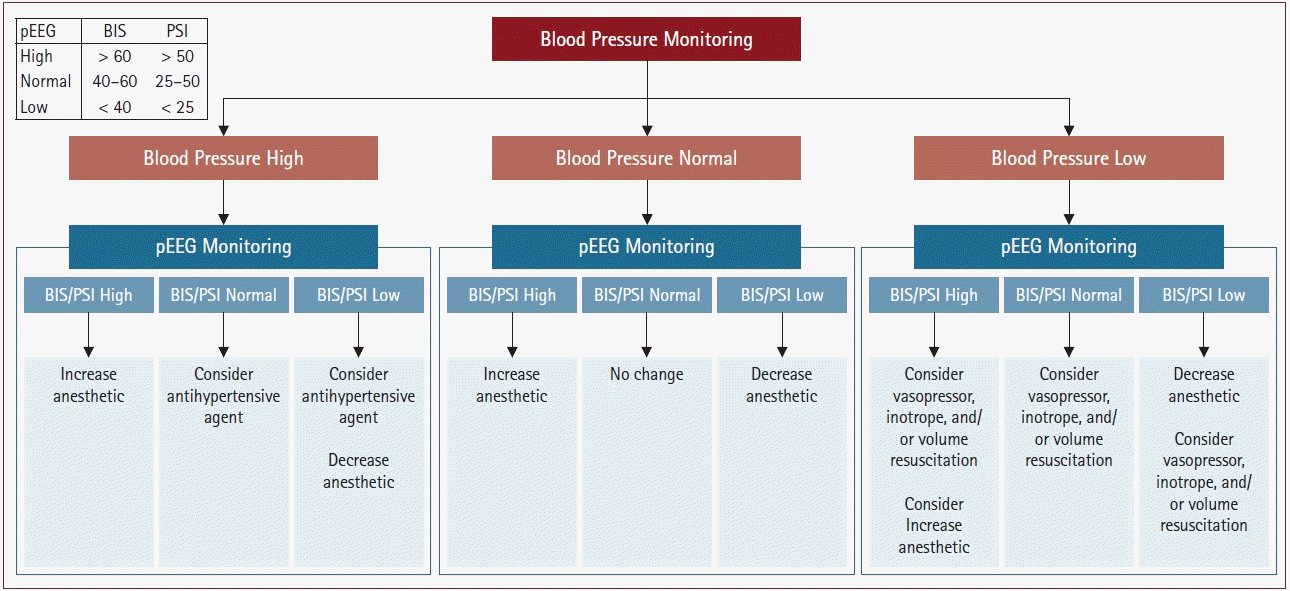
Table 1.
| Study name | Experimental design | Results | Conclusions |
|---|---|---|---|
| Cognitive dysfunction after anesthesia | · Demographics: aged ≥ 60, ASA ≥ 3 (16%), 61% male | · Median BIS values in the pEEG-guided and routine care groups were 53 and 36, respectively | · The authors estimate that for every 1,000 elderly patients undergoing major surgery, titration to a BIS between 40 and 60 prevented 23 and 83 patients from developing POCD and POD, respectively. |
| · Non-cardiac and non-neurosurgical | · pEEG-guided anesthesia reduced the amount of propofol and inhaled anesthetic drugs by 21% and 30%, respectively. | ||
| Chan et al. 2013 [47] | · Anesthetic technique: 11% TIVA & 89% inhaled agents | · Primary outcome: At three months, there was a 4.5% reduction in the incidence of POCD in the pEEG-guided group compared to the routine care group. | |
| · pEEG-guided group: target BIS 40–60 in 462 patients | · Secondary outcome: One week after surgery, there was no difference in the incidence of POCD, but an 8.5% decrease in the incidence of POD. | ||
| · Routine care in 459 patients. | |||
| · Primary outcome: incidence of POCD 3 months after surgery. | |||
| · Secondary outcome: incidence of POCD and POD one week after surgery. | |||
| Monitoring depth of anesthesia decreases the rate of POD but not POCD | · Demographics: aged ≥ 60, ASA ≥ 3 (48%), 54% male | · Mean average BIS values in the pEEG guided and routine care group were 39.0 and 38.7 respectively | · Intraoperative pEEG monitoring may provide anesthesia care providers with a tool to influence one of many factors that lead to POD. |
| · Non-cardiac surgery | · Primary outcome: 16.7% developed POD in the BIS-guided group and 21.4% in the routine care group. No difference in POCD was found between the groups | ||
| Radtke et al. 2013 [48] | · Anesthetic technique: 35% TIVA & 65% inhaled agents | · pEEG-guided anesthesia reduced the occurrence of extremely low BIS values (< 20) and burst suppression, possibly decreasing the incidence of POD. | |
| · pEEG-guided group: target BIS 40–60 in 575 patients | |||
| · Routine care in 580 patients. | |||
| · Primary outcome: POD & POCD at baseline, 1 week, and 3 months post-operation. | |||
| Postoperative delirium in a sub-study of cardiothoracic surgical patients in the BAG-RECALL clinical trial | · Demographics: aged ≥ 60, ASA ≥ 3 (100%), 63% male | · Primary outcome: 19% in the BIS group and 28% in the end-tidal-guided group developed POD, a 9% non-significant reduction in the BIS-guided group. | · A larger RCT is warranted to further explore the role of pEEG-guided versus end-tidal-guided anesthesia delivery on POD after cardiothoracic surgery. |
| · Cardiothoracic surgery | · Independent predictors of POD included low anesthesia dose, intraoperative transfusion, & ASA physical status. | · Patients in poor health may be more sensitive to anesthesia and have a higher risk of developing POD. | |
| Whitlock et al. 2014 [13] | · Anesthetic technique: inhaled agents | · Given the association between POD and poor patient outcomes, development of methods to minimize POD should be prioritized. | |
| · pEEG-guided group: target BIS 40–60 in 149 patients | |||
| · End-tidal-guided group: target between 0.7 and 1.3 MAC in 161 patients | |||
| · Patients screened twice a day for POD using the CAM-ICU | |||
| · Primary outcome: incidence of POD | |||
| The ENGAGES trial | · Demographics: aged ≥ 60, ASA ≥ 3 (34%), 54.3% male | · Primary outcome: 26% in the pEEG group and 23% in the routine care group developed POD. | · pEEG-guided anesthesia delivery did not decrease the incidence of POD compared with routine care. |
| · Major surgery (cardiac and non-cardiac) | · Intraoperative measures: median end-tidal anesthetic concentration was lower in the pEEG-guided group (0.69 vs. 0.80 MAC). Median duration of EEG suppression was less in the pEEG-guided group (SR > 1%: 7 vs. 13 min, BIS < 40: 32 vs. 60 min). No differences in MAP < 60 mmHg (7 min for both groups). | · Median end-tidal anesthetic concentration and median cumulative time with EEG suppression was significantly less in the pEEG-guided group. | |
| Wildes et al. 2019 [11] | · Anesthetic technique: inhaled agents | · Adverse events: No reported awareness with recall in either group. Death within 30 days: < 1% in the pEEG-guided group and 3% in the routine care group. | |
| · pEEG-guided group (BIS ≥ 40): 614 patients | |||
| · Routine care: 618 patients | |||
| · Patients screened for POD using the CAM-ICU | |||
| · Primary outcome: incidence of POD on postoperative days 1–5 | |||
| · Intraoperative measures: end-tidal anesthetic concentration, duration of EEG suppression (SR > 1% & time with BIS < 40), and hypotension (MAP < 60 mmHg). | |||
| · Adverse events: awareness with recall, death within 30 days. |
ASA: American Society of Anesthesiologists, BIS: bispectral index, TIVA: total intravenous anesthesia, pEEG: processed electroencephalography, POCD: postoperative cognitive dysfunction. Assessed using the selected tools. Some examples include the cognitive failure questionnaire to indicate potential subjective problems with perception, memory, and motor function, and neuropsychological tests, such as the verbal fluency test, verbal learning test, and color trail making test; POD: postoperative delirium. Assessed using the selected tools. Examples include the CAM-ICU; BAG-RECALL: BIS or Anesthesia Gas to Reduce Explicit Recall. This was a prospective, multi-center, randomized controlled trial to determine whether a bispectral index-guided protocol is superior to an anesthesia gas-guided protocol in reducing intraoperative awareness with explicit recall in high-risk surgical patients, RCT: randomized clinical trial, MAC: minimum alveolar concentration, CAM-ICU: Confusion Assessment Method for the intensive care unit, ENGAGES: Electroencephalography Guidance of Anesthesia to Alleviate Geriatric Syndromes, SR: suppression ratio, MAP: mean arterial pressure.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download