Abstract
Background
Giant lip hemangioma is a rare disease that may cause difficulty in preoxygenation and ventilation when using face masks and intubation during general anesthesia induction.
Case
A laparoscopic cholecystectomy was planned for a 77-year-old woman. The patient had a giant lower lip hemangioma that was 12 x 5 x 5 cm, which made preoxygenation and ventilation through a face mask impossible and put her at risk of hemangioma rupture. We preoxygenated her through a high-flow nasal cannula (HFNC). Following propofol and succinylcholine administration, we intubated the patient with a video laryngoscope without desaturation, hemangioma rupture, or CO2 retention.
Hemangioma is the most common benign tumor of vascular origin. Hemangiomas located around the airway can significantly increase the difficulty of endotracheal intubation as they occupy space and might rupture [1,2].
High-flow nasal cannula (HFNC) supplies a high flow of warm, humidified air or oxygen to the nasopharynx, reducing re-breathing and dead space, which in turn reduces CO2 retention [3].
We report on a patient with giant lower lip hemangioma who was expected to have a difficult airway and who seemed to be impossible to preoxygenate and ventilate with a face mask. We successfully preoxygenated the patient through HFNC to induce anesthesia without desaturation.
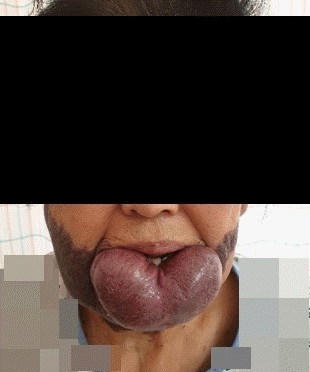
A 77-year-old female patient who was 153 cm tall and weighed 58 kg was admitted to hospital with chronic diarrhea. Abdominal and pelvis computed tomography showed that she had cholangitis due to gallbladder stones. The patient had a congenital giant hemangioma of the lower lip (Fig. 1). She had a history of hypertension and showed elevated liver enzymes. Other preoperative laboratory results, including coagulation tests, were within the normal range.
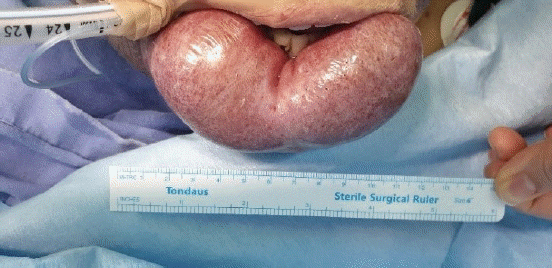
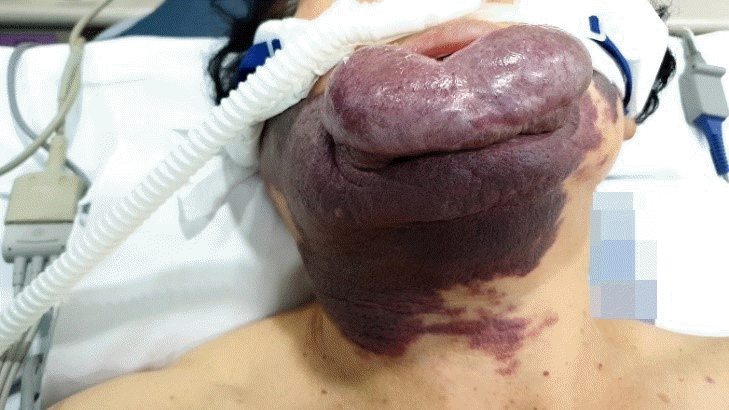
Laparoscopic cholecystectomy was planned. Due to the giant hemangioma of the lower lip, airway management was expected to be difficult because it seemed like a mask would not fit. The hemangioma was 12 × 5 × 5 cm (Fig. 2). Its surface was dry with no visible bleeding, was soft when pressed, and did not occupy the oral cavity. Manual displacement to the caudad was possible and the lower gum was well exposed. The mouth opening was two fingers wide and we classified the patient as class 2 Mallampati. Neck range of motion, jaw translocation, and thyromental distance were normal. The day before surgery, we explained the pros and cons of various airway management methods and the possible need for invasive airway access and got written informed consent. The written informed consent of publication for the use of photos and case details in this report has been obtained. Initial vital signs checked in the operation theatre showed that her blood pressure was 156/77 mmHg, heart rate was 66 beats/min, and oxygen saturation was 97%. Intraoperative monitoring devices were a radial artery catheter to monitor arterial blood pressure, an electrocardiogram, pulse oximeter, a BIS A-2000TM bispectral index monitor (Aspect Medical System, USA), and a train of four scans (IDMED, France). An HFNC Optiflow THRIVE (Fisher & Paykel Healthcare, New Zealand) with an FiO2 of 1.0 and a flow of 50 L/min was used for preoxygenation (Fig. 3). During 6 min of preoxygenation, we instructed the patient to breathe through her nose with her mouth closed. Arterial blood gas analysis after preoxygenation showed that her PaO2 level was 462 mmHg and PaCO2 level was 34 mmHg. To avoid bleeding due to the desiccation and friction, we covered the hemangioma with wet gauze to keep it moisturized. Difficult airway trolley, including emergency cricothyroidotomy kit and suction device were prepared. Anesthesia induction was performed using 1% propofol and succinylcholine. During airway manipulation, HFNC was maintained with an FiO2 of 1.0 and a flow of 50 L/min. An assistant applied a backward-upward-rightward-pressure maneuver to assist in visualizing the glottis while also manually displacing the hemangioma caudad. A cuffed endotracheal tube with an internal diameter of 7.0 mm was intubated with a stylet using a video laryngoscope (KoMAC Co., Ltd., Korea). While advancing the blade of the laryngoscope, we avoided it coming into contact with the hemangioma. The vocal cord was well visualized and classified as Cormack-Lehane grade 1 even though the tip of the blade did not reach the epiglottis. Intubation was successful without desaturation or damaging the hemangioma. After connecting the ventilator, the first end-tidal CO2 was 36 mmHg 118 s after propofol administration and analysis of arterial blood gas sampled right after confirmation of proper endotracheal tube position showed that the patient’s PaO2 level was 375 mmHg and PaCO2 level was 44 mmHg. Anesthesia was maintained with desflurane and remifentanil and 20 mg of rocuronium was administered at the start of surgery. At the end of the operation, HFNC was applied, all anesthetics were stopped, and muscle relaxation was reversed with sugammadex. The extubation and the recovery were uneventful. The patient was discharged at post-operation day 3.
A hemangioma is a malformation of vascular structures that is at risk of rupture. Its incidence rate is higher in females, Caucasians, premature infants, twins, and infants born from elderly mothers. The most common site is the head and neck region (60%) and followed by the trunk (25%) and limbs (15%). Of these, hemangiomas occurring in the lip area are classified as either superficial, deep, or mixed according to their depth. The patient in this case had a mixed type, which accounts for 49% of hemangiomas. Among lip hemangiomas, 60% occur in the upper lip, 36% in the lower lip, and 6% were in commissure form [1].
In this case, we used HFNC to preoxygenate and avoid hypoxia and intubated the patient using a video laryngoscope. The lesion was on the lower lip and it was large, so a mask would not fit, limiting the use of airway maintenance devices, including oropharyngeal airways. Without an airway-securing device, patients are prone to be hypoxic during anesthesia induction. There were also high risks of bleeding and aspiration if the hemangioma was damaged during airway manipulation. Bleeding may have hindered the clear visualization of anatomical structures, making intubation even more challenging [2]. As a result, we decided that it was important to secure sufficient apnea time. Therefore, we needed to be sure that the patient was preoxygenated well. Thus, we needed a device capable of supplying high FiO2 regardless of the patient’s inspiratory flow. An airway assessment with modified LEMON(Look externally, Evaluate 3-3-2 rule, Mallampati, Obstruction scores, Neck mobility) criteria indicated that intubation with a video laryngoscope seemed possible. We considered intubating the patient with a fiberoptic bronchoscope while she was awake. However, this procedure usually takes more time than intubation with a video laryngoscope. Moreover, sedation and analgesics used during fiberoptic bronchoscopy may cause hypoventilation leading to desaturation, which is grave when bag-mask ventilation is impossible. Furthermore, the chances of damaging the hemangioma would be higher when manipulating a fiberoptic bronchoscope, which we were less familiar with than a laryngoscope. If bleeding occurred, the fiberoptic bronchoscope would have been harder to use because the blood would severely limit visibility. Furthermore, the patient refused awake intubation because she was worried about the discomfort.
HFNC has many advantages over conventional oxygenation devices. HFNC supplies high oxygen flow and can warm and humidify it regardless of the high flow. Humidification enhances the washout of secretion and avoids mucosal injury. A high flow of oxygen reduces dead space because it decreases re-breathing and it generates positive end-expiratory pressure, which is higher with the mouth closed and is proportional to the flow. HFNC washes out CO2 during apneic oxygenation [3]. There have been reports of various procedures being completed without CO2 retention in a mean apnea time of 22.5 min using only HFNC with a FiO2 of 1.0 and a flow of 40–70 L/min without endotracheal intubation [4]. HFNC also allowed us to maintain oxygenation while attempting orotracheal intubation because it does not occupy the oropharynx where the intubation occurs.
However, the use of HFNC is not well established in any difficult airway guidelines. Studies comparing HFNC with conventional devices are ongoing. It may be suitable for use for ventilation in a patient with severe hypoxemic respiratory failure, preoxygenation during general anesthesia, intubation support, post-extubation support, oxygenation during bronchoscopy in a patient with a high risk of respiratory decompensation, and ventilation in laryngologic surgery [3].
There is controversy about whether HFNC can be used as a preoxygenation and ventilation device as an alternative to conventional face masks to induce general anesthesia. Studies show that apneic oxygenation through HFNC has a longer apnea time than apneic oxygenation through a face mask [5]. Also, when HFNC was used for preoxygenation and apneic oxygenation was continued until the airway was secured, the median apnea time, which was the time until O2 saturation falls below 90% after neuromuscular blockade was 14 min [6]. Furthermore, a study has shown that preoxygenation through HFNC increases operator convenience and reduces submandibular pain during induction [7]. Two studies are being conducted to determine whether HFNC can replace face masks during general anesthesia preoxygenation [8,9]. Taken together, this evidence shows that it may be possible to use HFNC in some patients instead of face masks for preoxygenation during general anesthesia induction.
We searched and reviewed cases in which face mask ventilation was not possible due to facial lesions and alternative methods were used. Full-text reviews were conducted for six articles [10-15] (Table 1). Our search strategy is presented in the Appendix. Of these articles, preoxygenation was performed using supraglottic airway device (SAD) in four cases [10-13], a Rendell-Baker-Soucek mask in one [14], and HFNC in one [15]. Regarding the use of SAD during preoxygenation after mild sedation while the patient is breathing on their own, inserting and removing an SAD for intubation might damage the hemangioma. Rendell-Baker-Soucek mask was not suitable for this patient due to the size of the hemangioma and might have pressed on the lesion, causing injury and bleeding.
In conclusion, we successfully managed a giant lower lip hemangioma patient for whom preoxygenation through a face mask seemed ineffective and bag-mask ventilation seemed difficult using HFNC. HFNC can be useful when difficult intubation is expected in patients who would have difficulty using a conventional face mask.
Notes
Author Contributions
Ji Yeon Kim (Supervision; Writing – original draft)
Hangaram Kim (Writing – original draft)
Min Hee Heo (Writing – review & editing)
Kyung Woo Kim (Writing – review & editing)
Sang-Il Lee (Writing – review & editing)
Kyung-Tae Kim (Writing – review & editing)
Jang Su Park (Writing – review & editing)
Won Joo Choe (Writing – review & editing)
Jun Hyun Kim (Conceptualization; Supervision; Writing – review & editing)
References
1. George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014; 18(Suppl 1):S117–20.

2. Murillo M, Zarza M, Gómez S, Chica J, Carrasquilla R, de la Vega J, et al. Anesthesia for emergency tracheostomy due to bleeding hemangioma of the tongue in a pregnant patient. Colomb J Anesthesiol. 2012; 40:313–7.

3. D’Cruz RF, Hart N, Kaltsakas G. High-flow therapy: physiological effects and clinical applications. Breathe (Sheff). 2020; 16:200224.
4. Gustafsson IM, Lodenius Å, Tunelli J, Ullman J, Jonsson Fagerlund M. Apnoeic oxygenation in adults under general anaesthesia using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) - a physiological study. Br J Anaesth. 2017; 118:610–7.

5. Hua Z, Liu Z, Li Y, Zhang H, Yang M, Zuo M. Transnasal humidified rapid insufflation ventilatory exchange vs. facemask oxygenation in elderly patients undergoing general anaesthesia: a randomized controlled trial. Sci Rep. 2020; 10:5745.

6. Patel A, Nouraei SA. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015; 70:323–9.

7. Tremey B, Squara P, De Labarre H, Ma S, Fischler M, Lawkoune JD, et al. Hands-free induction of general anesthesia: a randomised pilot study comparing usual care and high-flow nasal oxygen. Minerva Anestesiol. 2020; 86:1135–42.

8. Vourc’h M, Huard D, Feuillet F, Baud G, Guichoux A, Surbled M, et al. Preoxygenation in difficult airway management: high-flow oxygenation by nasal cannula versus face mask (the PREOPTIDAM study). Protocol for a single-centre randomised study. BMJ Open. 2019; 9:e025909.

9. Jo JY, Kim WJ, Ku S, Choi SS. Comparison of preoxygenation with a high-flow nasal cannula and a simple mask before intubation during induction of general anesthesia in patients undergoing head and neck surgery: study protocol clinical trial (SPIRIT Compliant). Medicine (Baltimore). 2020; 99:e19525.
10. Neeta S, Rao R, Upadya M, Keerthi P. Arteriovenous malformation of face: a challenge to anesthesiologists. Anesth Essays Res. 2017; 11:784–6.

11. Asai T, Matsumoto H, Shingu K. Awake tracheal intubation through the intubating laryngeal mask. Can J Anaesth. 1999; 46:182–4.

12. Nafiu OO, Coker N. A rather unconventional use of the laryngeal mask airway. Paediatr Anaesth. 2007; 17:998–1000.

13. Saini S, Dayal M, Gupta A. Infantile cystic hygroma: an unusual perioperative course. Anesth Essays Res. 2017; 11:268–70.

14. Saini S, Bansal T. Anesthetic management of difficult airway in a patient with massive neurofibroma of face: utility of Rendell Baker Soucek mask and left molar approach for ventilation and intubation. J Anaesthesiol Clin Pharmacol. 2013; 29:271–2.

15. Gusti V, Vaghadia H. Pre-oxygenation with Optiflow THRIVETM (transnasal humidified rapid insufflation ventilatory exchange) in a patient with impossible bag mask ventilation due to large facial tumor. J Clin Anesth. 2020; 64:109847.
Table 1.
Cases Using Alternative Preoxygenation Devices due to Difficult Face Masks Fitting
| Authors | Age (yr)/Sex | Type of lesion | Preoxygenation method | Intubation method | Mallampati/Comack-Lehan | Size of lesion |
|---|---|---|---|---|---|---|
| Neeta et al. [10] | 65/F | AVM of nose | SAD (i-gel) | Direct laryngoscopy | 2/N/A | 7 × 7 cm |
| Asai et al. [11] | 53/F | Post gangrenous mass | SAD (Fastrach) | Awake FOI through SAD | N/A/N/A | 5 × 5 cm |
| Nafiu and Coker [12] | 17/M | Intraoral mass | SAD (N/A) | SAD in situ | N/A/N/A | N/A |
| Saini and Bansal [13] | 1/M | Cystic hygroma | SAD (Proseal) | Direct laryngoscopy | N/A/N/A | 12 × 10 cm |
| Saini et al. [14] | 28/F | Neurofibroma | Rendell-Baker-Soucek | Direct laryngoscopy | N/A/N/A | N/A |
| Gusti et al. [15] | 68/M | Rhinophyma | HFNC | Videolaryngosopy | 2/1 | 12 × 7 cm |
Appendix
Appendix.
Search strategy
PUBMED, EMBASE
(‘difficult face mask’ or ‘difficult face mask’ or ‘difficult face mask’ or ‘difficult mask’ or ‘difficult airway’ or ‘impossible mask’ or ‘lip hemangioma’ or ‘face tumor’ or ‘face lesion’ or ‘perioral lesion’ or ‘perioral tumor’ or ‘face malformation’ or ‘circumoral tumor’ or ‘face deformity’) and (‘preoxygenation’ or ‘alternative airway’ or ‘laryngeal mask’ or ‘laryngeal airway’ or ‘HFNC’ or ‘High flow nasal cannula’ or ‘THRIVE’ or ‘transnasal humidified rapid insufflation ventilatory exchange’ or ‘rendell baker’ or ‘oxygen hood’ or ‘oxygen tent’ or ‘venturi mask’ or ‘non rebreather’ or ‘non rebreathing’)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download