Introduction
Pharmacokinetic principles for understanding volume kinetics
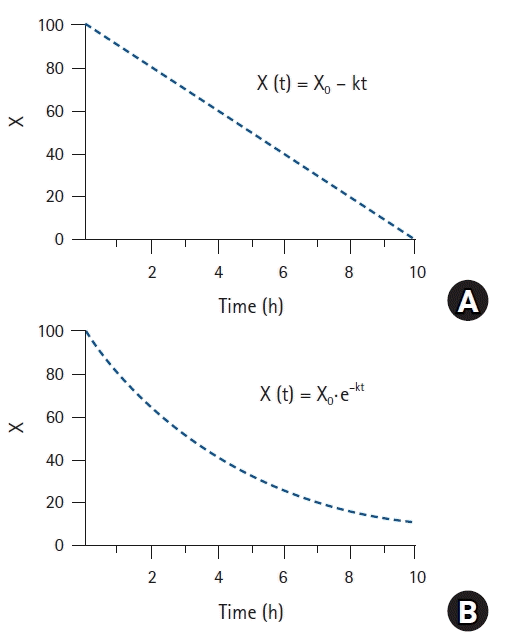
Kinetics of drugs
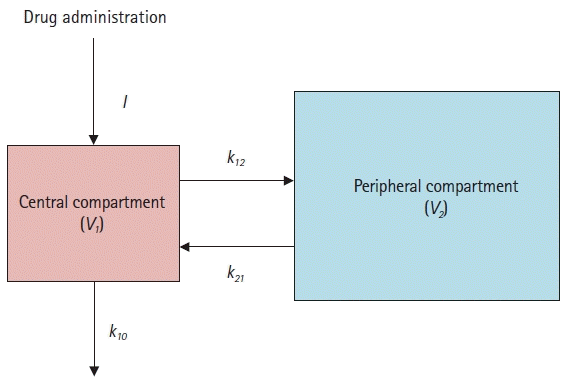
Mammillary compartmental model
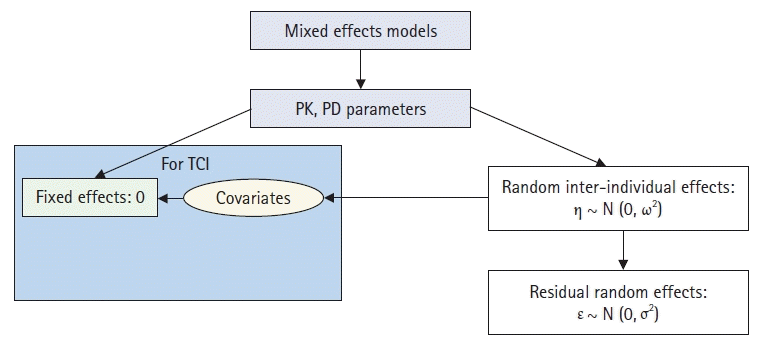
Population analysis
Calculation of plasma volume expansion caused by fluid administration using hemoglobin
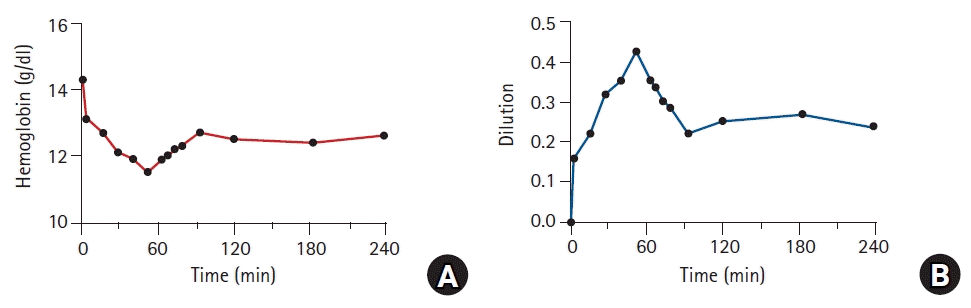 | Fig. 4.Changes in hemoglobin (A) and plasma dilution (B) caused by fluid administration. Male volunteer received 40 ml/kg Ringer’s lactate solution over 1 h. Dilution was calculated as follows:
where CHb(0) and Hct(0) are the hemoglobin concentration and hematocrit measured prior to the administration of Ringer’s lactate solution, respectively; CHb(t) is the hemoglobin concentration measured at any time t.
|
Structure model for volume kinetics
Table 1.
Dp(t): plasma dilution at any time t during intravenous infusion, CHb(0): hemoglobin concentration at t = 0 before intravenous infusion (g/dl), CHb(t): hemoglobin concentration at any time t after intravenous infusion (g/dl), Hct(0): hematocrit at t = 0 before intravenous infusion, Vp(0): plasma volume at t = 0 before intravenous infusion (ml), Vp(t): plasma volume at any time t after intravenous infusion (ml), Rate: infusion rate of the drug (mg/min), k10: elimination rate constant (1/min), A(t): drug amount at any time t (mg), V1: volume of distribution in the central compartment (ml), Cl: clearance (ml/min), V2: volume of distribution in the peripheral compartment (ml), Q: inter-compartmental clearance (ml/min), ki: infusion rate of fluid (ml/min), kb: basal elimination reflecting ongoing losses of water due to respiration, sweating, and basal renal filtration (ml/min), kr: renal clearance (ml/min), kt: distributional clearance (ml/min).
One-volume model
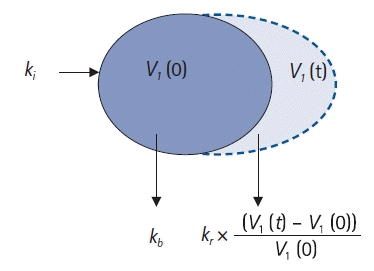 | Fig. 5.One-volume model. ki: infusion rate of the fluid (ml/min), kb: basal elimination reflecting ongoing losses of water due to respiration, sweating, and basal renal filtration (ml/min), kr: renal clearance (ml/min), V1(0): plasma volume at t = 0 before intravenous infusion (ml), V1(t): plasma volume at any time t after intravenous infusion (ml). |
Two-volume model
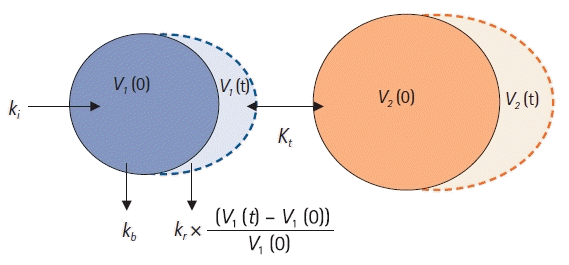 | Fig. 6.Two-volume model. ki: infusion rate of the fluid (ml/min), kb: basal elimination reflecting ongoing losses of water due to respiration, sweating, and basal renal filtration (ml/min), kr: renal clearance (ml/min), V1(0): plasma volume at t = 0 before intravenous infusion (ml), V1(t): plasma volume at any time t after intravenous infusion (ml), V2(0): peripheral volume at t = 0 before intravenous infusion (ml), V2(t): peripheral volume at any time t after intravenous infusion (ml), kt: distributional clearance (ml/min). |
Calculation of renal clearance using urine volume
Covariates describing inter-individual variabilities of volume kinetic parameters
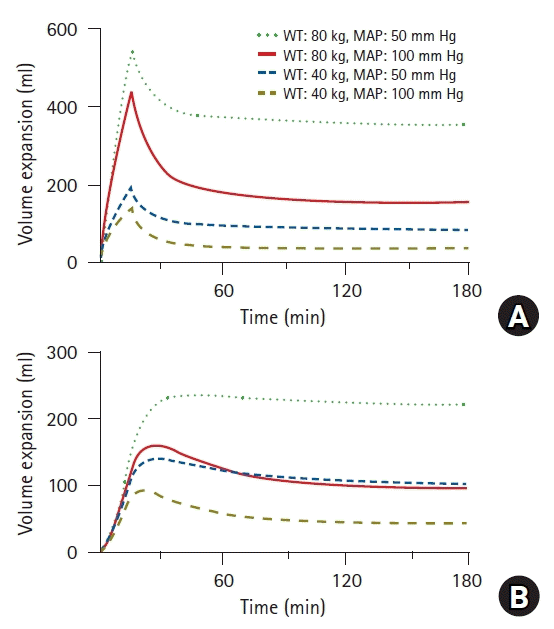 | Fig. 7.Simulated volume expansions of central (A) and peripheral (B) compartments in hypothetical patient receiving 10 ml/kg of Ringer’s lactate solution over 15 min followed by a rate of 8 ml/kg/h for 165 min. Four different cases were simulated based on weight (40 vs. 80 kg) and mean arterial pressure (50 vs. 100 mmHg). WT: weight, MAP: mean arterial pressure. |
Clinical application of volume kinetics
Limitations of fluid kinetics
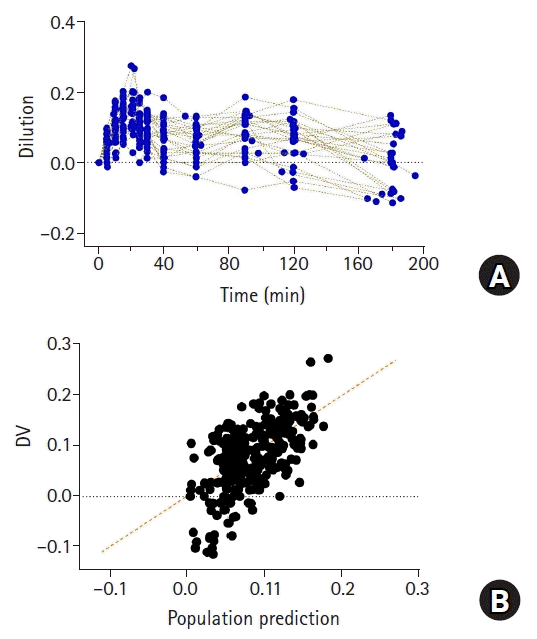 | Fig. 9.Plasma dilution (A) in 27 patients undergoing open gastrectomy. Patients were administered with 1,000 ml of Ringer’s lactate solution for 20 min, followed by continuous infusion at 6 ml/kg/h until time of last blood collection for volume kinetic analysis. Observed values (plasma dilutions) and population predicted values by volume kinetic model (B). Orange dashed line represents line of identity. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download