Introduction
The supraclavicular block (SCB) is widely used for intraoperative anesthesia and postoperative analgesia. The location of the lower trunk deep within the neural cluster, however, has raised concerns of ulnar nerve (UN) sparing when local anesthetic is not appropriately injected [
1,
2]. Incomplete blockade of the UN may be avoided by using the corner pocket (CP) approach during SCB [
3]. The target for needle tip placement is between the lateral or inferolateral side of the subclavian artery and the first rib. This approach provides high rates of successful blockade, rapid sensory onset, as well as a relatively short performance time [
4]. Unfortunately, even this approach cannot guarantee complete UN blockade [
4]. A previous study reported that injection into the perineural space through the sheath is required for a reliable block using the CP approach [
3]. Technical difficulties also exist, such as the need for significant caudal tilting of the ultrasound probe [
5]. This may cause difficultly of needle handling when using the in-plane technique, and anisotropy by angle of the insonating beam can result in poor image quality [
6]. Moreover, there is concern of pneumothorax due to the proximity of the needle and pleura [
7].
Recent improvements in ultrasound resolution have enhanced the ability to accurately identify and selectively block the three trunks of the brachial plexus [
2,
8], making the recently proposed intertruncal (IT) approach possible. This approach requires identification of the specific location of each individual trunk and the fine tissue planes between them [
9]. In the IT approach, local anesthetic is injected into the adipose tissue planes between the upper/middle trunk and the middle/lower trunk.
Although both approaches have been found to avoid UN sparing during SCB, no studies to date have directly compared the efficiency of UN blockade by the IT and CP approaches. We hypothesized that the IT approach (injection between lower and middle trunks) would result in a more complete blockade rate of the UN compared to the CP approach (injection below the lower trunk) at 15 min after blockade. The present study therefore compared the ulnar block characteristics of these two approaches.
Materials and Methods
The protocol of this prospective, parallel-arm, double-blind, randomized controlled superiority study was approved by the Institutional Review Board of Chungnam National University Hospital (CNUH 2020-05-070-001) and the trial was registered at the Clinical Research Information Service, a clinical trial registry in Korea (KCT0005268). This clinical research was done following the ethical principles for medical research involving human subjects in accordance with the Helsinki Declaration 2013. This study enrolled patients aged 20–70 years with American Society of Anesthesiologists physical status classification I and II and scheduled for elective forearm or hand surgery at Chungnam National University Hospital (Daejeon, Korea). All patients provided written informed consent. Patients were excluded if they refused to participate, had a local infection at the nerve block site, were hypersensitive to amide local anesthetic, had ipsilateral arm neuropathy, or had a history of neck surgery.
Study data were collected and managed using Research Electronic Data Capture (REDCap) software, a secure, web-based platform designed to support capturing of data for research studies and hosted at Chungnam National University Hospital (redcap.cnuh.co.kr) [
10]. This manuscript adheres to the applicable Consolidated Standards of Reporting Trials (CONSORT) guidelines [
11].
All blocks were performed by a single anesthesiologist (Y.J.) with experience in ultrasound guided regional anesthesia and under the direct supervision of the principal investigator (B.H.). Patients were randomly assigned at a ratio of 1 : 1 to the IT or CP group. Block randomization at sizes of 2 and 4 was performed using a random sequence generator (
www.randomization.com) [
12]. To conceal the allocation, the sequence was uploaded onto REDCap, allowing access only to the researcher performing the assigned block. All other individuals who participated in the surgery, including attending anesthesiologists, surgeon, nurses, and outcome assessors, were blinded to the group assignment.
Immediately prior to the block, each patient was administered 1 mg intravenous midazolam for pre-medication. All blocks were performed under ultrasound guidance using an in-plane technique with a high-resolution ultrasound system (X-Porte, FUJIFILM SonoSite, Inc., USA), a high frequency linear probe (HFL50xp: 15–6 MHz, X-Porte), and a nerve stimulator (0.1 ms, 0.5 mA, 2 Hz, sentinel mode, MultiStim SENSOR, PAJUNK, Germany). Each patient was injected with a total of 25 ml of a 1 : 1 mixture of 0.75% ropivacaine and 1% lidocaine using a 22 gauge, 80 mm, echogenic needle (SonoPlex cannulas, PAJUNK, Germany).
Patients were maintained in a supine position with the head turned to the contralateral side and the ipsilateral shoulder slightly elevated with a pillow. The needle was inserted lateral to the brachial plexus through the prevertebral fascia. In the IT group, the hyperechoic outer boundaries (epineurium) of each trunk were distinguished by ultrasound scanning. The optimal image for patients in the IT group was defined as an image with well differentiated middle and lower trunks. Efforts were not required to obtain a perfect CP view. The gap between the lower and middle trunks was confirmed, first by carefully injecting 0.5 ml of local anesthetic agents to open up the adipose tissue layer (hydrodissection), and subsequently by securing a safe route for needle advancement in the IT plane. While confirming that the trunk of the brachial plexus was not swollen, 10 ml of local anesthetic agents was slowly injected between the lower and middle trunks, 7.5 ml was injected between the middle and upper trunks, and the remaining 7.5 ml was injected between the upper trunk and prevertebral fascia (
Fig. 1A). In the CP group, the optimal image was obtained by placing the probe so that the entire subclavian artery was above the first rib. The needle tip was advanced between the lateral or inferolateral side of the subclavian artery and the first rib. While confirming negative blood aspiration, 10 ml of local anesthetic agents was slowly injected in the CP. The remaining 15 ml was injected in the planes identical to those in the IT group (
Fig. 1B).
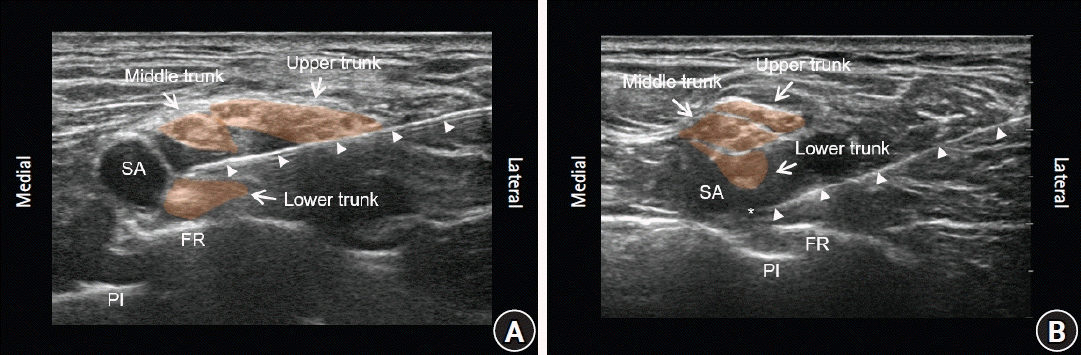
Fig. 1.
(A) Ultrasonography image during the IT approach. Note the needle (white arrow head) has penetrated the brachial plexus sheath, and its tip is lying in the IT layer. The middle trunk appeared to be floating on the injected local anesthetic drug. (B) Ultrasonography image during the CP approach. Injection of local anesthetic agents in the CP. *CP: corner pocket, FR: first rib, IT: intertruncal, SA: subclavian artery, Pl: pleura.

Sedation was induced using dexmedetomidine (loading dose 1 μg/kg for 10 min and maintenance dose of 0.2–0.5 μg/kg/h) and discontinued at the beginning of skin suture. Supplemental oxygen was administered prior to sedation at a rate of 5 L/min via a simple facial mask. Brachial plexus block (BPB) was considered successful when the surgery was completed without the need to inject additional local anesthetics into the surgical field, without the need to perform a rescue nerve block, and without conversion to general anesthesia. These decisions were completely at the discretion of the attending anesthesiologist. At the time of the BPB procedure, the procedure time, defined as the interval between needle insertion and removal, and the presence of the dorsal scapular artery (DSA) in the ultrasound scan images were recorded.
Immediately after the BPB procedure, the assigned, blinded outcome assessor, who was not present during the BPB procedure, measured patient satisfaction by asking the patients: How would you score your discomfort during the block on a scale of 0 to 10, where 0 indicates no discomfort and 10 indicates the worst discomfort imaginable. The same researcher assessed sensory block (pin-prick test) and motor block in the areas of the ulnar (UN), median (MN), musculocutaneous (MCN), and radial (RN) nerves every 5 min for at least 30 min until the blockade was complete. Sensory blockade was graded on a 10-point scale (normal = 10, absent = 0) relative to a pin-prick sensation in the contralateral arm. Sensory blockade of the UN, MN, MCN, and RN was assessed on the volar aspect of the fifth finger, the volar aspect of the thumb, the lateral aspect of the forearm, and the lateral aspect of the dorsum of the hand, respectively. Motor blockade was graded on a three-point scale (normal = 3, mildly reduced = 2, markedly reduced = 1, unable to move = 0). Motor blockade of the UN, MN, MCN, and RN was assessed by measuring thumb opposition with the little finger, thumb opposition with the index finger, elbow flexion, and wrist extension, respectively.
Sensory recovery in the UN territory was assessed every 30 min by the patients. The patients were instructed to repeatedly pinch the little finger of each hand and check the time of sensory normalization in the anesthetized hand by comparison with the opposite hand. The assessor visited the patient the following morning (within 24 h postoperatively) to ascertain the presence of residual blockade, neurologic deficits, and any other symptoms. Neurologic complications were evaluated again during an outpatient clinic visit 7 days after surgery. Postoperative chest radiography is a routine postoperative pathway of our institution and was used to identify accidental pneumothorax.
The primary outcome was the proportion of participants with complete sensory block of the UN 15 min after BPB. Complete sensory blockade of each nerve was defined as a pin-prick score of 0. Time to readiness for surgery was defined as the time required to achieve complete sensory block of the areas of all four nerves. Secondary outcomes included the proportion of patients with complete motor blockade, duration of the procedure, patient discomfort score during the procedure, incidence of noticing the DSA during the procedure, duration of sensory blockade of the UN, and sensory and motor scores as a function of time.
Statistical analysis
The sample size was calculated based on the primary outcome according to the superiority hypothesis [
13]. Based on our clinical experiences, about 40% of patients undergoing the CP approach had complete sensory blockade in the UN territory within 15 min after BPB. Based on the assumption that 80% of patients undergoing the IT approach would achieve complete anesthesia within 15 min, 27 subjects per group would have a power of 90% and a risk of 5% for type I errors. Based on a combined 10% rate of dropouts and data losses, 60 participants were enrolled.
All analyses were per protocol using R software version 4.0.0 (R Project for Statistical Computing, Austria). Normality of distribution of continuous variables was assessed using Shapiro–Wilk tests. Normally distributed continuous variables were reported as mean ± standard deviation (SD) and analyzed by independent sample t-tests, whereas non-normally distributed continuous variables were reported as median (interquartile range) and analyzed by Mann–Whitney U tests. Categorical variables were reported as number (%) and analyzed by χ2 or, when expected count was < 5, Fisher’s exact test. A two-tailed P value < 0.05 was considered statistically significant.
Results
Of 60 patients assessed for eligibility, 59 were enrolled and analyzed; one patient randomized to the IT group was excluded due to uncooperative outcome evaluation (
Fig. 2). The baseline characteristics of the participants are described in
Table 1. All patients successfully underwent ultrasound-guided SCB regardless of approach, and there were no complications directly related to the technique including pneumothorax or the use of local anesthetics.
Fig. 2.
CONSORT diagram showing the patients at every stage of the randomized controlled trial. IT: intertruncal, CP: corner pocket.
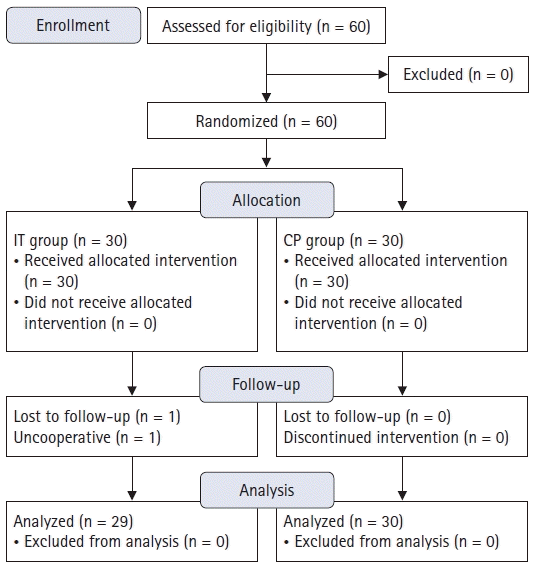

Table 1.
Demographic and Clinical Characteristics
|
Variable |
IT group (n = 29) |
CP group (n = 30) |
|
Age (yr) |
52.0 (27.0, 59.0) |
48.5 (26.0, 60.0) |
|
Sex (M/F) |
15/14 |
15/15 |
|
Height (cm) |
167.0 (157.0, 176.0) |
164.0 (154.0, 175.0) |
|
Weight (kg) |
65.0 (56.0, 80.0) |
62.5 (55.0, 74.0) |
|
Surgery time (min) |
60.0 (52.0, 80.0) |
64.0 (48.0, 82.0) |
|
ASA PS (I/II) |
9/20 |
9/21 |
|
Type of surgery (a/b/c/d) |
2/17/6/4 |
3/10/4/13 |

The rates of complete sensory blockade (75.9% [22/29] vs. 43.3% [13/30], P = 0.023) and complete motor blockade (82.8% [24/29] vs. 50.0% [15/30], P = 0.017) of the UN after 15 min were significantly higher in the IT than in the CP group (
Fig. 3). There were no between-group differences in rates of complete sensory and motor blockade in the four neural territories at each time point, with most patients achieving complete block within 20 min (
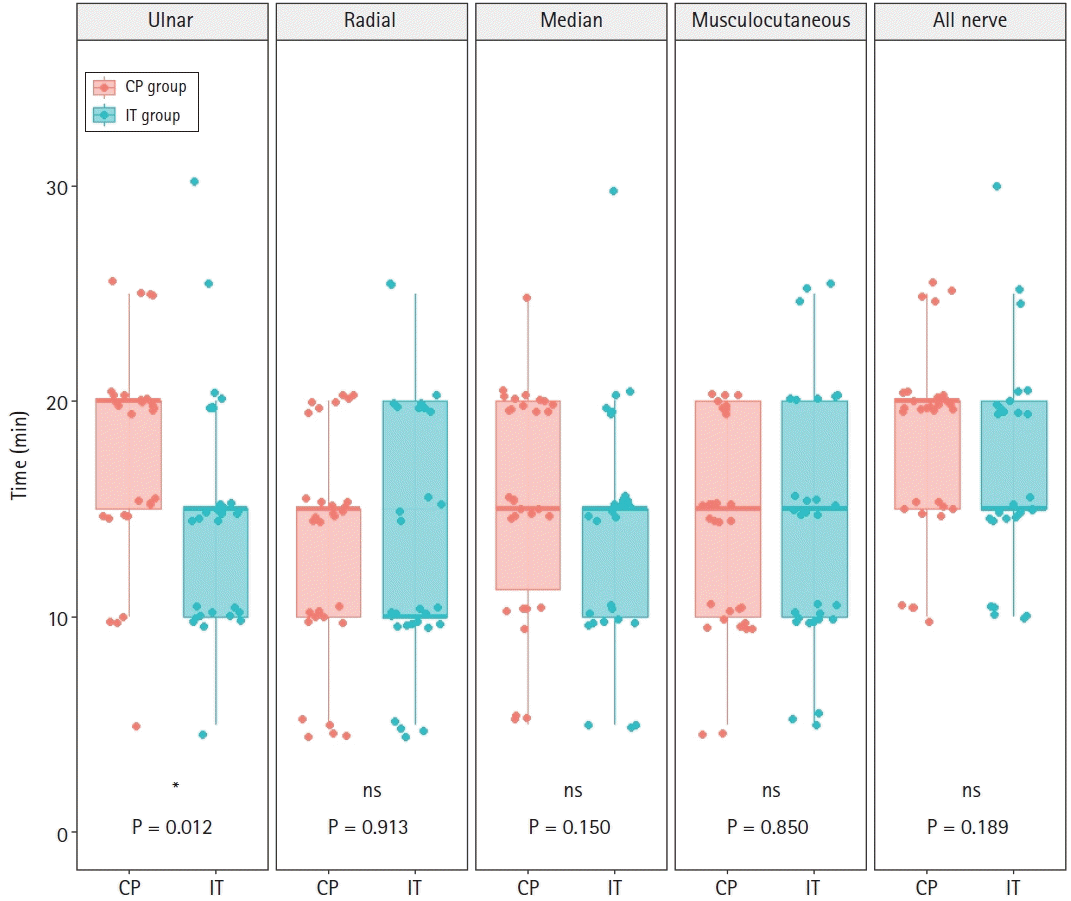
Fig. 3). Time to onset of sensory block in the UN was significantly shorter in the IT than in the CP group (15.0 [10.0, 15.0] min vs. 20.0 [15.0, 20.0] min, P = 0.012), but there was no difference between the IT and CP groups in time to onset of complete sensory blockade of all nerves (15.0 [15.0, 20.0] min vs. 20.0 [15.0, 20.0] min, P = 0.189) (
Fig. 4). The progression of sensory and motor blockade of the UN territory is shown in
Supplementary Fig. 1.
Fig. 3.
Proportions of patients with complete sensory (A) and motor (B) block according to time in distributions of the ulnar nerve. Proportions of patients with complete sensory (C) and motor (D) block according to time in distributions of the all four nerves (total). IT: intertruncal, CP: corner pocket. *P < 0.05.
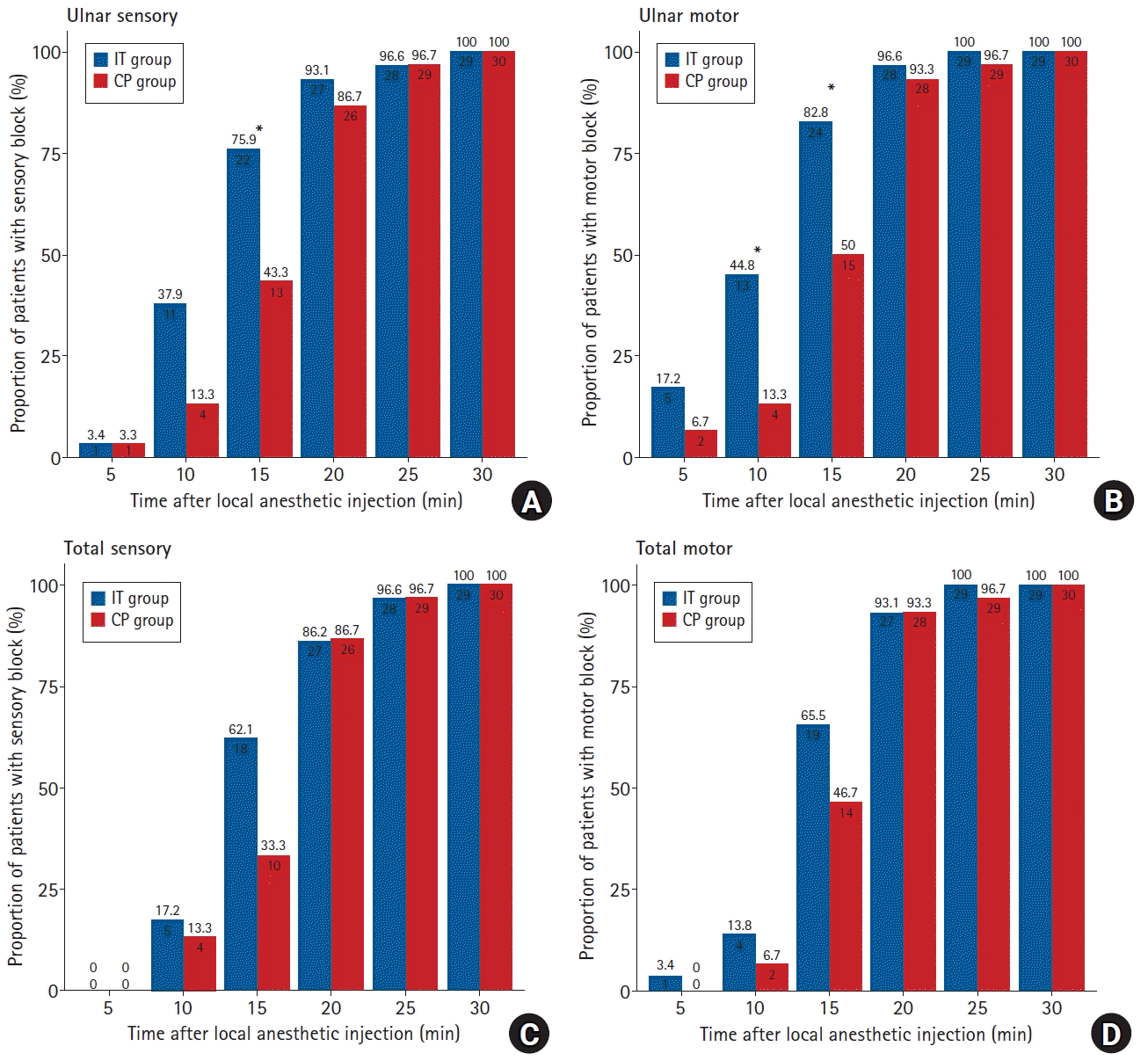

Fig. 4.
Onset times of sensory block of each nerve and all four nerves in the IT and CP groups. IT: intertruncal, CP: corner pocket, ns: not significant. *P < 0.05.

Total procedure duration and patient discomfort scores were not significant in the IT and CP groups. However, the DSA was only seen in ultrasonographic images in the 7 patients of IT group. Unlike the onset time, which was faster in the IT group, the total duration of UN sensory block did not differ in the IT and CP groups (
Table 2). None of the patients reported transient or persistent neurological signs or symptoms after 24 h or at the one-week follow-up after surgery.
Table 2.
Effects of IT and CP Blockade on Patient Outcomes
|
Variable |
IT group (n = 29) |
CP group (n = 30) |
P value |
|
Procedure time (s) |
250.0 (232.0, 277.0) |
268.0 (213.0, 299.0) |
0.834 |
|
Patient discomfort scale (0–10) |
3.0 (2.0, 6.0) |
5.0 (3.0, 6.0) |
0.304 |
|
Visualization of DSA |
7 (24.1) |
0 (0) |
0.014 |
|
Sensory block duration of UN (min) |
548.5 (476.0, 698.0) |
502.5 (433.5, 646.0) |
0.313 |

Discussion
UN sparing is a frequent limitation in the conventional approach to SCB when relying on blind techniques or nerve stimulated muscle contraction [
14]. Although use of the CP approach under ultrasound guidance was thought to avoid UN sparing, studies suggest that UN sparing may still occur [
4]. The present study found that, although both approaches were effective for UN block, the IT approach provided a faster onset of UN block.
Compared with extrafascial injection, subfascial injection was reported to induce a faster onset and prolonged duration of sensory and motor blockade without causing neurological complications [
15]. The slower onset of UN block in the CP group may have been due to the extrafascial spread of local anesthetics. Moreover, the IT approach directly penetrates the sheath, whereas the CP approach does not. Additionally, injecting local anesthetic above the lower trunk may facilitate a circumferential pattern of spreading [
16]. Our results suggest that the IT approach provides a faster onset of UN block than the CP approach. Also, the IT approach can be used as an alternative in cases where it is difficult to do the CP approach.
Early papers of ultrasound guidance described the brachial plexus as a ‘cluster of hypoechoic nodules’ or as a main and other satellite ‘neural clusters’ [
14,
17]. During targeted intracluster injection, the needle is advanced within the sheath containing one or more clusters of hypoechoic neurologic elements, and the local anesthetic is injected inside the clusters [
18,
19]. The nerve structures were approached as a group, without distinguishing the identity and location of each individual trunk due to relatively low qualities of ultrasound imaging. The only reported advantages of intracluster injection are faster onset times and time to surgical readiness [
9]. These advantages, however, may be offset by the risk of intraneural injection of local anesthetics, which may result in neuronal damage [
18]. The performance of bilateral intracluster blocks on cadavers resulted in 25% of specimens having subperineural ink on histologic examination, with 90% of the latter being intrafascicular with evidence of axonal distortion or damage [
20]. Intracluster injections may result in nerve injury due to unintended subperineural injection. The homogeneity of nerve elements, tightly compressed together in a small space, reduces generated echoes, such that the needle tip may not be clearly distinguishable within the cluster.
Recent advancements in sonographic resolution have led to the recognition that the original cluster was composed of individual trunks and/or divisions [
2]. Higher resolution equipment has enabled the identification of the specific location of each individual trunk and the fine tissue planes between them. This has allowed the application of the IT approach using hydrodissection between fine adipose tissue planes and the outer boundary of the epineurium that surrounds the fascicle without accidental intraneural injection [
9], which may overcome the disadvantages of the intracluster approach. In addition, concomitant use of a nerve stimulator further reduces the possibility of inadvertent neural complications, as the needle cannot come within too close a proximity to the nerve.
During proper injections, we observed the expansion of extraneural tissues and increases in the diameter of the entire complex under the fascia, while each trunk remained at its original size. For successful SCB, the block needle should penetrate the brachial plexus sheath and local anesthetic agents should be injected into the connective tissue matrix between the neural elements [
21]. However, it remains unclear whether subfascially injected anesthetics spread intraneurally [
22,
23]. More than 50% of the brachial plexus in the supraclavicular region is thought to be composed of fat and connective tissue inside the sheath [
16,
22]. These findings suggest that subfascially injected local anesthetics are not necessarily deposited in the fascicles but in the connective tissue matrix, resulting in a mere injection into the adipose layer. We also found that none of our patients experienced permanent neurological complications or delayed recovery from nerve damage. Although the external boundaries of each trunk were identified and nerve integrity was preserved using the IT approach, additional clinical and histological studies are needed to evaluate the safety of this approach.
To obtain the optimal image of the CP and avoid pleural puncture, the probe had to be tilted more caudally until the subclavian artery and brachial plexus were above the first rib, particularly when the brachial plexus was located more medially with respect to the first rib. An important advantage of the IT approach is that caudal tilting of the probe and deep injection were not absolutely required during lower trunk block. Despite these advantages, however, the DSA was observed in about 24% of patients in the IT group. In such cases, it was inevitable to tilt the probe caudally as in the CP approach to avoid arterial puncture. The DSA was identified as a branch of the subclavian artery that passed through the brachial plexus. A study assessing the presence of the DSA at three ultrasound probe positions commonly used in SCB found that the DSA passed most frequently (23/106, 21.7%) through a probe position in which the brachial plexus was on the first rib or was partially on the pleura and lateral to the subclavian artery, which lay directly on the pleura [
24]. This probe position is commonly used for the IT approach during SCB.
Another interesting point of our study is that unlike the previous study reporting UN sparing after the CP approach [
4], all patients showed complete UN block. This may be due to differences in needle positioning. While we confirmed floating of the lower trunk as an optimal image during CP injection, the injection point in the previous study was an approximately 1 cm
2 area bounded medially by the subclavian artery. Although it is difficult to distinguish intra or extra sheath injection on ultrasound image during CP injection, the difference in proximity to the lower trunk may explain our excellent results regarding UN block.
This study had several limitations. First, the success of the block was evaluated at 15 min, suggesting the need for caution when generalizing our results. The time setting in this study was based on the work flow in our institution. Second, all nerve blocks were performed by experienced anesthesiologists. Because the IT approach requires the use of hydrodissection to construct a path between the trunks to avoid neural injury, there is a need for a learning curve. Third, the sensory block duration time was determined based on self-reporting by patients, suggesting that the quality of the data may be relatively low.
In conclusion, the IT approach provides a more rapid onset of UN blockade than the CP approach and can be a good alternative for SCB. However, additional studies are required to ascertain the safety of the IT approach, especially in terms of neural damage.





