Abstract
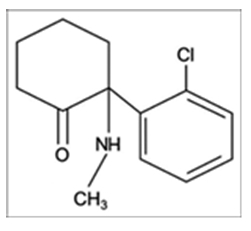
Initially known as CI-581, ketamine was first synthesized in 1962 as a replacement from phencyclidine. It has since been used as an anesthetic and analgesic. In addition, it has bronchodilating, sedative, and amnestic properties, preserving airway reflexes and sympathetic nervous system tone. Since the discovery of ketamine, it has been a major topic of discussion due to controversies regarding its usage in particular sets of patients. In the past 50 years, despite its potential benefits, it is not commonly used because of concerns of “emergence phenomenon,” its use as a substance of abuse, and its systemic side effects. Since 2012, three World Health Organization reviews on ketamine have addressed its international control.
Researchers have been studying this wonder drug for a decade worldwide. Many myths of ketamine regarding emergence phenomenon and its use in traumatic brain injury and open eye injury have been disproved in recent times. It is becoming popular in pre-hospital settings, critical care, emergency medicine, low-dose acute pain services, and adjuvant in regional anesthesia techniques. This review highlights the current consensus on the various applications of ketamine in the literature.
Go to : 
Ketamine is a versatile drug with a unique profile, permitting its worldwide usage in many situations. Its variable dosing regimens make it a wonderful agent for anesthesia induction at high dosages with preservation of protective reflexes, and a potent analgesic in low-dose infusions. With the advent of newer and considerably safer drugs and because of its problems of abuse, ketamine use has reduced. Recently, researchers worldwide have gained interest in ketamine, and studies have been conducted on this agent and are included in this review.
This article highlights the controversies related to psychosomatic issues caused by ketamine use. The authors also discuss the evidence and consensus regarding its use in patients with raised intracranial and intraocular pressures in acute and chronic pain services and critical settings.
Go to : 
1962: Calvin Stevens at Parke Davis laboratory synthesized a compound known as CL 581 from phencyclidine, which he later named ketamine.
1964: Its first use as anesthetic on prisoners in Michigan state prison by Dr. Corssen
1968: After Food and Drug Administration (FDA) approval, ketamine anesthesia was first used on American soldiers during the Vietnam War.
1970-80: Recreational use of ketamine became popular worldwide.
Early 1980s: Emergence phenomenon led to increasing illicit use and withdrawal from mainstream anesthetic use in humans.
2000: Antidepressant effects of ketamine used in resistant cases by Dr. Berman at Yale University.
2002: Continuous infusions became popular for intractable complex regional pain syndrome by Drs. Harbert and Correl at Yale University.
2013: Moved from Schedule H to Schedule X drug to prevent its use per Drugs and Cosmetics Act
2014: Effect of ketamine on suicidal ideation by Dr. Price at Yale University and as treatment of posttraumatic stress disorder
2015: Large scale persistent network reconfiguration demonstration by Dr. Yang
Go to : 
Ketamine is a phencyclidine derivative and a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist that blocks the phencyclidine sites on NMDA receptors mostly present in the cerebral cortex, thalamus, limbus, and spinal cord. This in turn leads to neuronal depolarization (Fig. 1). Its analgesic action is mainly due to its effects on opioid receptors. Added to the regular intravenous route, it can also be administered intramuscularly, intranasally, and interosseously.
It is highly lipid soluble and has a low protein binding capacity. It is metabolized primarily in the liver via hydroxylation and N-demethylation into Nor-ketamine, which is 30% as potent as ketamine with weak analgesic properties.
Go to : 
In patients with normal autonomic activity, it has a central sympathomimetic action leading to tachycardia, hypertension, and increased cardiac output. This is because it is an agent of choice for inductions in acute hypovolemic shock, whereas it is to be avoided in patients with coronary artery diseases. In patients with depleted catecholamines, such as in chronic shock and critically ill patients, it has a negative inotropic effect, which further accentuates shock-like states. If the autonomic activity is normal, sympathomimetic effects overrides negative inotropic action [1].
It preserves the airway tone and laryngo-pharyngeal reflex and causes bronchodilation. However, airway reflexes are very unpredictable in infants [2]. Intravenous ketamine use in large boluses could lead to transient apnea, as it may have a slight respiratory depressant effect and decrease the stimulant action of hypercarbia [3].
Ketamine also affects the metabolic and endocrine systems. It increases blood glucose, plasma cortisol, and prolactin levels [1]. It also leads to excessive salivation; thus, some clinicians advocate the routine use of an antisialagogue.
Since its discovery, ketamine has remained controversial because of associated dogmas. We wish to disprove the associated myths and establish evidence-based facts.
Go to : 
Elevated intracranial pressure (ICP) with ketamine is one of the biggest controversies regarding the use of ketamine in patients with a head injury. It is a notion that ketamine can cause a rise in ICP through sympathetic stimulation, potentially exacerbating the condition. However, when ketamine is used with a γ-aminobutyric acid (GABA) agonist, this rise in ICP may not occur [4]. Furthermore, by increasing cerebral perfusion, ketamine may benefit patients with neurological injury. A thorough literature search found a series of six studies published in the 1970s that reported an association between ketamine and increased ICP. Moreover, these studies were case reports and small case-control studies. These publications were confounded by patients with abnormal cerebrospinal fluid pathways, which included patients with aqueductal stenosis and obstructive hydrocephalus [5]. None of these studies have directly evaluated the effect of ketamine in patients with traumatic brain injuries (TBIs). Unfortunately, the myth that ketamine was contraindicated in TBIs has persisted until recently when large-scale studies showed conflicting results. A recent study has directly studied the effects of ketamine on ICP in patients with TBI and showed that ketamine is one of the best agents to facilitate airway management in patients with a head injury [6]. A large systematic review based on Cochrane methodology with Oxford level 2b, GRADE C evidence also supports that ketamine does not increase ICP in sedated and ventilated patients with severe TBI, and ketamine may lower the ICP in some patients [7]. In patients with TBI, it is important to maintain the mean arterial pressure, prevent hypoxia and hyperventilation, and mitigate increases in ICP, and ketamine helps fulfill these conditions. Ketamine retains the patient's respiratory drive, does not decrease the blood pressure, yields no increase in ICP, and allows for an additional advantage over other sedation medications and behavioral control without apnea [8]. Recent publication has provided evidence for the role of ketamine in neuroprotection [9]. In addition, ketamine can be administered intramuscularly or through the intranasal route, and therefore, it can be used easily in emergency settings [10]. Lastly, ketamine has a high therapeutic index that allows flexibility in dosing, which is necessary to obtain an accurate body weight [11].
The initial work on ketamine and its effects on intraocular pressure (IOP) was performed by Corssen and Hoy in 1967, who described an increase in IOP; however, the study had several limitations [12]. The study included patients of different ages undergoing general anesthesia for various surgeries. They included only 15 pediatric patients, many of whom had incomplete data. The pre-medications used in the study were not standardized and included agents not generally used in the pediatric age group for this purpose (e.g., barbiturates). The investigators studied the patients for 3 min after drug administration, a time point that alone is not appropriate for the pharmacokinetics of this drug. In almost half of the subjects, the measurements either did not change or decrease in one or both eyes. A lesser proportion of IOP elevation was noted in pediatric cases, and these subjects yielded a pooled mean increase of less than 3 mmHg from control readings. A landmark study by Drayna et al. [13] proved that at dosages ≤ 4 mg/kg, there are no clinically meaningful associations of ketamine with IOP. Recently, a systematic review comprising nine studies including 293 patients also suggested that the administration of ketamine to pediatric patients during ocular tonometry has little or no effect on IOP. Two out of the nine studies mentioned a slight rise in IOP if ketamine doses were more than 4 mg/kg [14]. Further studies are warranted to determine whether ketamine can be used safely for procedural sedation when elevated IOP or globe injury is a concern.
Consensus: Available literature suggest that ketamine influences the IOP in a dose-dependent manner. Generally, doses less than 4 mg/kg do not lead to an increase in IOP and at times decreases it, but most analyses have a low level of evidence and further large trials are needed to validate it the dose dependent influence of ketamine on IOP.
The analgesic characteristics of ketamine are primarily established via NMDA receptors located in the central nervous system [15] and to some extent through opioid receptors [16]. The American Society of Regional Anesthesia and the American Society of Anesthesiologists have published an entire consensus on the role of ketamine in acute pain management. They have supported the use of ketamine for acute pain in a variety of contexts, including as a stand-alone treatment, as an adjunct to opioids and especially in opioid-tolerant patients. They have recommended that ketamine bolus doses should not exceed 0.35 mg/kg and infusions for acute pain should not exceed 1 mg/kg/h in settings where there is no intensive monitoring [17]. Ketamine's adverse effects will prevent some patients from tolerating higher doses in acute pain settings, and unlike in chronic pain therapy, lower doses (i.e., 0.1–0.5 mg/kg/h) may be needed to achieve an adequate balance of analgesia and adverse effects (grade C recommendation, moderate level of certainty). Consensus guidelines also state that clinicians should exclude or limit ketamine use in patients with commonly considered contraindications. These include severe hepatic dysfunction (cirrhosis) [18] and high-risk coronary artery disease [19]. A recent meta-analysis also reported that patients receiving continuous sub-anesthetic ketamine experienced considerably less pain than those treated with traditional opioid regimens [20].
Another Cochrane systematic review of 37 randomized controlled trials (RCTs) also concluded that sub-anesthetic doses of ketamine are effective in reducing morphine requirements in the first 24 h after surgery, and it also reduces postoperative nausea and vomiting, which is the main problem with opioids, the most commonly used formulations for postoperative analgesia [21]. It has been studied not only for intravenous use in perioperative analgesia but also by various other routes. Feltracco et al. [22] showed that epidural infusion of sub-anesthetic doses of S(+)-ketamine during thoracic surgery provides better postoperative analgesia than epidural ropivacaine. Preemptive intranasal ketamine (1.5 mg/kg) enhances postoperative analgesia after endoscopic nasal surgery [23]. Ketamine spray (0.5 mg/kg) in the tonsillar fossa is effective for post-tonsillectomy pain control in children [24]. A recent RCT evaluating low-dose S(+)-ketamine in target-controlled intravenous anesthesia with remifentanil and propofol for open gynecological surgery concluded that there was no effect on the 24-h cumulative morphine consumption with S(+)-ketamine; however, there was a delayed emergence from general anesthesia reported with S(+)-ketamine [25].
Consensus: The application of ketamine at sub-anesthetic doses in the perioperative setting has been associated with reduced pain scores, opioid requirements, and postoperative nausea and vomiting, without any considerable side effects. Moreover, good results have been established using ketamine for surgery patients with high levels of postoperative pain. The level of evidence is still poor to recommend it as a sole agent for perioperative infusions for analgesia, although a good level of evidence is present for its use as an adjuvant.
The action of ketamine on opiate tolerance and hyperalgesia combined with its direct analgesic activity has prompted its increasing use in chronic pain states [8]. IV ketamine has also been evaluated in phantom limb pain, and a study reported that infusion of 300 μg/kg in 60 mL solution over 3 h led to complete remission [26]. Low-dose intranasal S(+)-ketamine ketamine is beneficial for the ad hoc treatment of breakthrough pain in patients with neuropathic pain [27]. Oral ketamine has a poor safety profile and its efficacy in chronic pain management discourages its use, but its use may be limited as an add-on therapy in patients with complex chronic pain when other therapeutic options have failed [28]. However, the long-term effectiveness of ketamine in treating chronic pain remains controversial, as studies often demonstrate contradicting results. A recent meta-analysis comprising six clinical trials in which ketamine was compared with a placebo during chronic non-cancer pain found moderate evidence suggesting the efficacy of ketamine during chronic pain. However, it was found that ketamine increased the incidence of psychedelic manifestations in comparison to placebo. Further studies are warranted in this regard so as to determine optimal administration regimes of this agent during this condition [29]. Studies conducted by Connolly et al. [30] (meta-analysis of six studies in 2015), Maher et al. [31] (review of 11 RCTs in 2017), and Michelet et al. [32] (review of six RCTs in 2018) suggest that there is no high-quality evidence available evaluating the efficacy of ketamine for complex regional pain syndrome and all manuscripts examined in this review were of moderate-to-low quality.
Ketamine is now considered to be an essential adjuvant analgesic for refractory cancer pain, and it is on the World Health Organization list of essential drugs for patients who no longer respond to high doses of opioids or have predictable breakthrough pains [33]. Nowadays, ketamine is regarded as an essential adjuvant drug in palliative care in many countries. It can be administered in various regimens through oral, intravenous, intrathecal, subcutaneous, and topical routes of administration [34]. A recently published Cochrane systematic review studying ketamine as an adjuvant to refractory cancer pain concluded that the current evidence is insufficient to assess the benefits and adverse effects of ketamine as an adjuvant to opioids. Furthermore, dose escalation to as high as 500 mg did not lead to any added clinical advantage and rather may have serious adverse events [35]. However, a 2012 Cochrane review on the use of ketamine in cancer pain found two RCTs that suggest the use of ketamine as an adjunct to morphine because it improves the effectiveness of morphine in cancer pain. This review included 32 case reports, of which 28 reported excellent pain relief with ketamine. The adverse effects commonly reported were sedation and hallucinations, although they were not severe [35]. One dose-escalation, double-blind, randomized, placebo-controlled phase III trial including 185 participants, in which ketamine or the placebo was delivered subcutaneously over 3 to 5 days, concluded that ketamine does not have a net clinical benefit when used as an adjunct to opioids and standard co-analgesics in cancer pain [36]. A literature search found four RCTs that examined the benefit of oral, subcutaneous, or intravenous ketamine in opioid refractory cancer pain, and none showed clinically relevant benefits in relieving pain or reducing opioid consumption [37].
Ketamine has been proven to be an extremely effective treatment for major depression, bipolar disorder, and suicidal behavior. Resistance to regular antidepressants is a growing concern in patients with manic depressive disorder. The slow onset and moderate degrees of receptor occupancy by ketamine could largely be used to avoid the anesthesia effect, dissociation, and psychotomimetic reactions [38]. Ketamine works incredibly fast, relieving depression in less than 2 h, which is unlike conventional anti-depressants that generally take weeks to work [39,40]. A systematic review also showed ketamine to be a rapid and effective treatment option for depression, reducing suicidal ideation, with minimal short-term side effects [41]. In 2018, Chen et al. [42] found that 0.5 mg/kg dose of ketamine infusion was beneficial for the cognitive function of patients with resistant depression.
Ketamine is a racemic mixture with equal parts of R(-)‐ketamine and S(+)-ketamine. Of the two, S(+)-ketamine was developed as an antidepressant agent owing to its higher affinity for NMDA receptors. On March 5, 2019, S(+)-ketamine nasal spray was approved by the US FDA for the same purpose [43].
Although the efficacy of ketamine has also been shown in depressed patients with a history of psychotic symptoms, its administration in patients with psychotic disorders has largely been neglected due to its potential to exacerbate dissociative or psychotic symptoms [44].
A study by Duman and Li [45] showed that a single injection of ketamine increased prefrontal synaptogenesis and reversed stress-induced atrophy. This is consistent with findings that mice carrying a mutant form of glycogen synthase kinase 3, an enzyme involved in synaptic plasticity, are unresponsive to ketamine [46].
Research on ketamine is still preliminary, and many facets remain obscured. For instance, there is no consensus regarding its effects on controlled long-term use. Most studies have been conducted on drug abusers in a correlational manner, and thus generalization to “physically healthy” human population is difficult. As most studies enrolled many disproportionately young patients, it is questionable whether results can be translated to more vulnerable depressed populations (e.g., elderly patients and patients with cardio-vascular impairments) [47].
(1) Premedication: Ketamine can be administered orally (5–8 mg/kg), intramuscular (4–6 mg/kg), or IV (1–2 mg/kg) routes. The advantages include its analgesic properties and the ability to cause sedation without respiratory depression [48]. Because of its rapid onset of action, ketamine has been used as an IM induction drug in children and patients with intellectual disabilities [49]. Intranasal ketamine is widely studied for procedural sedation. A recent publication reviewed 11 studies and suggested that intranasal ketamine at doses up to 10 mg/kg appears to be safe in children with adequate analgesia at doses as low as 0.5 mg/kg. They also concluded that the most common adverse effect of intranasal ketamine was vomiting, reported in ten studies at doses of at least 1 mg/kg. Although studies on children suggested that adequate sedation can be achieved safely with an intranasal route, evidence is limited and the overall quality of evidence is low, necessitating the need for larger high-quality trials [50]. It is used for sedation or general anesthesia for pediatric procedures such as cardiac catheterization, radiation therapy, radiological studies such as magnetic resonance imaging, dressing changes, and dental work [51]. Combination of low doses of ketamine and propofol provides effective and safe sedation-analgesia in pediatric emergency short surgical procedures and in adults undergoing colonoscopy and short gynecological procedures [52–54].
(2) Caudal epidural block: A quantitative systematic review of RCTs on adding ketamine to pediatric caudal anesthesia concluded that ketamine prolonged analgesia with few side effects compared with local anesthetic alone [55]. Another meta-analysis stated that caudal ketamine in pediatric patients was associated with decreased post-operative pain and non-opioid analgesic requirements [56]. In 2018, Aliena et al. [57] compared the postoperative analgesic effect of caudal bupivacaine with and without ketamine in pediatric sub-umbilical surgeries and found that caudal administration of ketamine 0.5 mg/kg along with 0.75 ml/kg 0.25% bupivacaine significantly prolonged the duration of postoperative analgesia in children more than plain bupivacaine, without any significant adverse reactions.
Adjuvants used with ketamine to averse its undesirable effects are known, but very recently, there is an increasing amount of evidence suggesting its utility as an adjuvant to averse the effects of sedatives such as dexmedetomidine. In 2012, Tobias [58] in his review highlighted that dexmedetomidine is generally effective for sedation during noninvasive procedures but there is enough literature available challenging its efficacy as a sole agent for painful procedures. Its disadvantages include its long onset time, limited analgesic effect, and undesirable hemodynamic perturbations such as bradycardia and hypotension. Tobias categorically mentioned many robust trials where adding low-dose ketamine to dexmedetomidine led to satisfactory outcomes in invasive procedures in both adults and children. He suggested that when used together, ketamine prevents bradycardia and hypotension, which has been reported with dexmedetomidine alone, and also hastens the onset. Char et al. [59] clearly stated that dexmedetomidine alone is inadequate to provide adequate sedation for either pediatric intensive care management or procedural sedation. It also has an adverse effect on the conduction system, leading to hemodynamic instability. They observed a decrease in heart rate after dexmedetomidine administration, which returned to baseline after co-administration of ketamine (mean difference between baseline and after ketamine 6.5 bpm; 95% CI, 11.2 to 1.8; P = 0.005). In 2020, a double-blind randomized RCT concluded that adding ketamine to dexmedetomidine led to good postoperative analgesia, decreased postoperative opioid requirements, and led to smooth recovery after functional endoscopic sinus surgery [60]. Sinha et al. [61] compared combination of dexmedetomidine and ketamine with dexmedetomidine alone for awake fiberoptic nasotracheal intubation and concluded that the addition of low-dose ketamine (15 mg as a bolus of 5 ml, followed by continuous infusion at 20 mg/h) further enhanced hemodynamic stability and provided better sedation than dexmedetomidine alone. Kim et al. [62] and Chun et al. [63] in their respective studies have found that a combination of ketamine and dexmedetomidine led to shorter procedural time, improved sedation quality, and quicker onset of sedation with fewer incidences of desaturation. Qiao et al. [64] reported a lower rate of successful venous cannulation in children with dexmedetomidine alone (47%), whereas it was 80% when ketamine was added (P = 0.006).
Ketamine used in patients in a critical care unit provides combined sedation and analgesia, has favorable effects on hemodynamics, and can treat persistent bronchospasm. A recently published multi-centric retrospective study evaluated the use of ketamine infusions in 390 adult intensive care unit (ICU) patients and suggested that there is no consensus regarding the indication, dose, or dose units associated with ketamine. Hemodynamic changes are common and start occurring as early as 4 h after the infusion; however, other adverse effects appear to be minimal [65]. In 2015, Umunna et al. [66] also suggested that ketamine is a good sedative agent along with its low incidence of adverse effects; thus, it may be a reasonable alternative for patients requiring mechanical ventilation. In septic patients with cardiovascular instability, ketamine reduces inotropic support and exerts a protective anti-inflammatory effect against the sepsis process because of its cardiovascular stimulatory effects [67].
Overall, very little research is available on its use in critical care settings and its efficacy in the emergency department and anesthesia practice has been extrapolated to its applicability in ICUs.
Ketamine offers a plausible pharmacologic mechanism for use in the setting of severe alcohol withdrawal as both ketamine and alcohol antagonize the NMDA receptor. The authors of a retrospective study assessed the effect of ketamine infusion added to the usual therapy with benzodiazepines titrated to symptoms on requirements for benzodiazepines in patients admitted with delirium tremens and found that the rate of endotracheal intubation and mean duration of ICU stay were drastically reduced [68]. However, another retrospective analysis noted that benzodiazepine doses drastically decreased after initiation of a ketamine infusion. In addition, better symptom control was achieved with a new regimen, which had no adverse neurological outcomes or hemodynamic perturbations [69].
Unfortunately, very limited literature is available for ketamine use in alcohol withdrawal, and all studies were retrospective, open-label studies involving the simultaneous use of numerous different agents. None of these studies proved anything about the use of ketamine infusions for alcohol withdrawal
Super-refractory status epilepticus (SRSE) is defined as status epilepticus (SE) continuing after 24 h of anesthetic infusion or recurring after discontinuation of the anesthetic infusion. Typically, SE is treated with a combination of benzodiazepines and specific antiepileptics, quickly escalating to anesthetic infusions (most commonly propofol). Prolonged SE and SRSE are associated with a downregulation of GABA-A receptors and an upregulation of NMDA receptors, placing ketamine as a therapeutic adjunct, especially due to concerns about the adverse effects of prolonged propofol infusions, such as hyperlipidemia and propofol infusion syndrome. There are no randomized trials comparing ketamine to other agents in SRSE. Sabharwal et al. [70] studied patients, most of whom were on both propofol and ketamine infusions, in a neurocritical care setting. They concluded that 1.5–10.5 mg/kg/h is the recommended dose of ketamine infusions and should be combined with a 1.5–8 mg/kg/h dose of propofol infusion. A case audit involving several countries revealed that ketamine was considered only for the most severe cases of SE very late in the course of the disease [71]. More recent experiences with ketamine and the possibility for a neuroprotective effect support the early administration (duration of SE < 3 days) rather than the late rescue [72,73]. An Italian group reported their experience with children on a protocol that used IV ketamine for refractory SE upfront added to other antiepileptics. They found that in instances in which ketamine was used as the sole anesthetic agent for refractory SE, there was the added benefit of avoiding endotracheal intubation [74]. This experience has not been replicated in adults but is intriguing. In 2015, Sreshtha et al. [75] also concluded that ketamine can be a safe, effective, readily available, and economic therapeutic agent for the management of SRSE in patients with hemodynamic instability.
Unfortunately, no RCT is available to test its safety and efficacy in patients with SE. Most of the available studies are either case reports or series with a low level of evidence.
Most of these clinical applications have just been mentioned in case reports with no randomized trials. Large multi-centric studies are needed to recommend its routine use.
Several opportunities remain for ketamine to be studied in cardiac surgery patients. The current literature supports the idea that ketamine attenuates the inflammatory response to cardiac surgery with CPB. Whether this consistently leads to improved clinical outcomes remains unclear. It also has a potential use in the post-cardiac surgery ICU because of its excellent hemodynamic profile, minimal respiratory depression, and potent analgesic properties. However, at this time, there is a paucity of studies to support its use in this setting. Mogahad et al. [76] compared the safety and efficacy of ketamine-dexmedetomidine (KD) with that of ketamine-propofol (KP) combinations for sedation in patients after coronary artery bypass graft surgery and concluded that KD provided a short duration of mechanical ventilation with less fentanyl dose requirement in comparison with KP, whereas hemodynamic stability and length of the ICU stay were the same for both regimens. Future studies comparing ketamine to other commonly accepted sedative regimens, such as propofol and dexmedetomidine, are critically needed [77]. Some authors have reported that a single dose of ketamine 0.5 mg/kg administered upon induction was associated with lower serum levels of C-reactive protein and lower incidence of delirium and cognitive dysfunction after cardiac surgery with CPB. This is because of the neuroprotective and anti-inflammatory effects of ketamine [78].
Given the need for anesthesia during electroconvulsive therapy (ECT) and the excitement about ketamine's acute effects in reducing depressive symptoms, combining the two therapies seems to be a logical next step. A study showed that S(+)-ketamine administered for ECT decreased the number of ECT sessions, produced lower depression severity scores, and higher cognitive ratings [79]. A study showed that the ketamine-propofol combination (ketofol) can be an alternative strategy to enhance the seizure quality and clinical efficiency of ECT [80,81]. Shams and El-Masry [82] compared KD with KP in the ECT procedure and concluded that KD had an effective anti-depression effect and resulted in less agitation and more patient satisfaction than KP.
However, the research on ketamine use in ECT has been disappointing [83]. The clinical enthusiasm is tempered by concerns that ketamine's antidepressant activity is short-lived and by the uncertainty regarding the long-term safety in repeated administrations, with unknown risks for long-term cognitive side effects, psychotic symptoms, and substance abuse [84].
Go to : 
Most anesthesiologists are apprehensive of hallucinations, delirium, and nightmares that patients experience while awakening, and these clusters of symptoms are categorized as the "reemergence phenomenon." Several receptors as well as neurochemical mechanisms have been hypothesized to be implicated in the occurrence of the emergence phenomena (EP) such as NMDA, opiates, dopamine, and acetylcholine. Hence, a wide variety of drugs belonging to different classes are being tested to prevent or treat symptoms of the EP with some success.
The 37th expert committee on drug dependence released a review report, which also states that these symptoms were reduced by the concurrent use of benzodiazepines, putting the patient in a low-stimulus environment and providing information on the possible emergence reactions preoperatively.
A RCT was conducted in 2011 on 200 patients who received ketamine for procedural sedation. The authors concluded that 23% of patients experienced a reemergence phenomenon with ketamine, whereas only 8% of patients exhibited such symptoms when 0.03 mg/kg of midazolam was administered [89]. Another cross-sectional observational study conducted by Lohit et al. [90] also concluded that the perioperative administration of midazolam with ketamine was effective in controlling EP, leading to a smooth postsurgical recovery. Another recent RCT published in 2019 also concluded that premedication with either midazolam 0.05 mg/kg or haloperidol 5 mg intravenously significantly reduces ketamine-induced recovery agitation while delaying recovery [91].
Ketamine uropathy describes the effects of ketamine on the urinary system. It is a misnomer to use ketamine cystitis or ketamine bladder syndrome as while the bladder is often most severely affected, the whole urinary tract may be damaged. A 2019 study from Taiwan found that 84% of the 106 ketamine users had shown lower urinary tract symptoms (LUTS), and in most of these cases, LUTS appeared at a mean of 24 months after its daily use [92]. The largest study assessed symptoms in 1,056 male users and noted a prevalence of 76%. Again, they found a significant correlation between higher age (> 30 years), longer duration of use (> 24 months), and the co-use of other illicit drugs with symptom severity [93]. Chang et al. [94] in their study reported moderate-to-severe LUTS including frequency, urgency, dysuria, and hematuria when the mean daily consumption of ketamine was 3.2 ± 2.0 g and they also reported that the mean interval from consumption to the development of LUTS was 12.7 months (range, 2–36 months).
The authors could not find any large RCT on ketamine uropathy, but all available literature clearly stated that the long-term continuous use of ketamine (most commonly as recreational agent) leads to LUTS, which are mostly dose- and duration-dependent. However, most of these symptoms are reversible after the discontinuation of ketamine.
Go to : 
Ketamine’s role in the operating room as an anesthetic, sedative, amnestic, and analgesic agent has been well established since its discovery. A moderate level of evidence is available to prove its role in TBI patients, acute pain services, chronic non-cancer pain, and as an antidepressant. A low level of evidence is available for its role in patients with raised IOP (dose < 4 mg/kg), cancer pain management, and critical care settings. Most of its adverse reactions are minor, and the incidence of emergence reaction has been reported in most of the available literature but can be easily managed with the concurrent use of benzodiazepines.
Currently, there are several ongoing trials despite the increasing addiction burden in the Western society. However, various policies, legislations, and support groups are coming up to fight against the addiction problems with ketamine, popularly known as the “super K.” In the era of evidence-based medicine, the so-called “super K” can be a boon to medical science if we leave our age-old fears of conquering the heights. This drug is both good and bad. Now, the challenge is to find the pious and fight the evil.
Go to : 
Notes
Author Contributions
Abhijit Kumar (Conceptualization; Data curation; Formal analysis; Software; Visualization; Writing – original draft)
Amit Kohli (Conceptualization; Data curation; Formal analysis; Software; Visualization; Writing – original draft; Writing – review & editing)
Go to : 
References
1. Best W, Bodenschatz C, Beran D. World Health Organization Critical Review of Ketamine. 36th WHO Expert Committee on Drug Dependence report, 6.2. Geneva: World Health Organization; 2014. Report No.: WHO technical report series no 991.
2. Dolansky G, Shah A, Mosdossy G, Rieder M. What is the evidence for the safety and efficacy of using ketamine in children? Paediatr Child Health. 2008; 13:307–8.
4. Himmelseher S, Durieux ME. Revising a dogma: ketamine for patients with neurological injury? Anesth Analg. 2005; 101:524–34.

5. Filanovsky Y, Miller P, Kao J. Myth: Ketamine should not be used as an induction agent for intubation in patients with head injury. CJEM. 2010; 12:154–7.

6. Hughes S. Is ketamine a viable induction agent for the trauma patient with potential brain injury. BestBETs [Internet]. 2011 Nov 30 [cited 2020 Dec 17]. Available from www.bestbets.org/bets/bet.php?id=2180.
7. Zeiler FA, Teitelbaum J, West M, Gillman LM. The ketamine effect on ICP in traumatic brain injury. Neurocrit care. 2014; 21:163–73.

8. Kurdi MS, Theerth KA, Deva RS. Ketamine: Current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014; 8:283–90.

9. Hudetz JA, Pagel PS. Neuroprotection by ketamine: a review of the experimental and clinical evidence. J Cardiothorac Vasc Anesth. 2010; 24:131–42.

10. Vallerand AH, Sanoski CA, Deglin JH. Davis's drug guide for nurses. 15th ed. Philadelphia: F.A. Davis Company;2016.
11. Murray MJ. Faust's anesthesiology review. 4th ed. Philadelphia: Elsevier Saunders;2015. p. 166.
12. Corssen G, Hoy JE. A new parenteral anesthetic-CI-581: its effect on intraocular pressure. J Pediatr Ophthalmol Strabismus. 1967; 4:20–3.

13. Drayna PC, Estrada C, Wang W, Saville BR, Arnold DH. Ketamine sedation is not associated with clinically meaningful elevation of intraocular pressure. Am J Emerg Med. 2012; 30:1215–8.

14. Ruiz-Villa JO, Jaramillo-Rivera DA, Pineda-Gutierrez LM. Ketamine impact on intraocular pressure of children: a systematic review and qualitative synthesis of evidence. Colomb J Anesthesiol. 2019; 47:226–35.
15. Cohen SP, Liao W, Gupta A, Plunkett A. Ketamine in pain management. Adv Psychosom Med. 2011; 30:139–61.

16. Niesters M, Aarts L, Sarton E, Dahan A. Influence of ketamine and morphine on descending pain modulation in chronic pain patients: a randomized placebo-controlled cross-over proof-of-concept study. Br J Anaesth. 2013; 110:1010–6.

17. Schwenk ES, Viscusi ER, Buvanendran A, Hurley RW, Wasan AD, Narouze S, et al. Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018; 43:456–66.

18. Bell RF. Ketamine for chronic noncancer pain: concerns regarding toxicity. Curr Opin Support Palliat Care. 2012; 6:183–7.
19. Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1989; 36:186–97.

20. Kator S, Correll DJ, Ou JY, Levinson R, Noronha GN, Adams CD. Assessment of low-dose i.v. ketamine infusions for adjunctive analgesia. Am J Health Syst Pharm. 2016; 73(5 Suppl 1):S22–9.

21. Bell RF, Dahl JB, Moore RA, Kalso EA. WITHDRAWN: Perioperative ketamine for acute postoperative pain. Cochrane Database Syst Rev. 2015; (7):CD004603.
22. Feltracco P, Barbieri S, Rizzi S, Ori C, Groppa F, De Rosa G, et al. Brief report: perioperative analgesic efficacy and plasma concentrations of S+ -ketamine in continuous epidural infusion during thoracic surgery. Anesth Analg. 2013; 116:1371–5.
23. Abdel-Ghaffar HS, Salem MA. Safety and analgesic efficacy of pre-empitive intranasal ketamine versus intranasal fentanyl in patients undergoing endescopic nasal surgery. J Am Sci. 2012; 8:430–6.
24. Hosseini Jahromi SA, Hosseini Valami SM, Hatamian S. Comparison between effect of lidocaine, morphine and ketamine spray on post-tonsillectomy pain in children. Anesth Pain Med. 2012; 2:17–21.

25. Ithnin FB, Tan DJ, Xu XL, Tan CH, Sultana R, Sng BL. Low-dose S+ ketamine in target-controlled intravenous anaesthesia with remifentanil and propofol for open gynaecological surgery: a randomised controlled trial. Indian J Anaesth. 2019; 63:126–33.

26. Shanthanna H, Huilgol M, Manivackam VK. Early and effective use of ketamine for treatment of phantom limb pain. Indian J Anaesth. 2010; 54:157–9.

27. Huge V, Lauchart M, Magerl W, Schelling G, Beyer A, Thieme D, et al. Effects of low-dose intranasal (S)-ketamine in patients with neuropathic pain. Eur J Pain. 2010; 14:387–94.

28. Blonk MI, Koder BG, van den Bemt PM, Huygen FJ. Use of oral ketamine in chronic pain management: a review. Eur J Pain. 2010; 14:466–72.

29. Michelet D, Brasher C, Horlin AL, Bellon M, Julien-Marsollier F, Vacher T, et al. Ketamine for chronic non-cancer pain: A meta-analysis and trial sequential analysis of randomized controlled trials. Eur J Pain. 2018; 22:632–46.

30. Connolly SB, Prager JP, Harden RN. A systematic review of ketamine for complex regional pain syndrome. Pain Med. 2015; 16:943–69.

31. Maher DP, Chen L, Mao J. Intravenous ketamine infusions for neuropathic pain management: a promising therapy in need of optimization. Anesth Analg. 2017; 124:661–74.
32. Michelet D, Brasher C, Horlin AL, Bellon M, Julien-Marsollier F, Vacher T, et al. Ketamine for chronic non-cancer pain: a meta-analysis and trial sequential analysis of randomized controlled trials. Eur J Pain. 2018; 22:632–46.
33. Benítez-Rosario MA, Salinas-Martín A, González-Guillermo T, Feria M. A strategy for conversion from subcutaneous to oral ketamine in cancer pain patients: effect of a 1:1 ratio. J Pain Symptom Manage. 2011; 41:1098–105.

34. Abdollahpour A, Saffarieh E, Zoroufchi BH. A review on the recent application of ketamine in management of anesthesia, pain, and health care. J Family Med Prim Care. 2020; 9:1317–24.

35. Bell RF, Eccleston C, Kalso EA. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst Rev. 2012; 11:CD003351.

36. Hardy J, Quinn S, Fazekas B, Plummer J, Eckermann S, Agar M, et al. Randomized, double-blind, placebo-controlled study to assess the efficacy and toxicity of subcutaneous ketamine in the management of cancer pain. J Clin Oncol. 2012; 30:3611–7.

37. Jonkman K, van de Donk T, Dahan A. Ketamine for cancer pain: what is the evidence? Curr Opin Support Palliat Care. 2017; 11:88–92.

38. Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016; 37:865–72.

39. Reardon S. Rave drug tested against depression. Nature. 2015; 517:130–1.
40. Salvadore G, Singh JB. Ketamine as a fast acting antidepressant: current knowledge and open questions. CNS Neurosci Ther. 2013; 19:428–36.

41. Reinstatler L, Youssef NA. Ketamine as a potential treatment for suicidal ideation: a systematic review of the literature. Drugs R D. 2015; 15:37–43.

42. Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM, et al. Cognitive function of patients with treatment-resistant depression after a single low dose of ketamine infusion. J Affect Disord. 2018; 241:1–7.

43. Hashimoto K. Rapid-acting antidepressant ketamine, its metabolites and other candidates: a historical overview and future perspective. Psychiatry Clin Neurosci. 2019; 73:613–27.

44. Bartova L, Papageorgiou K, Milenkovic I, Dold M, Weidenauer A, Willeit M, et al. Rapid antidepressant effect of S-ketamine in schizophrenia. Eur Neuropsychopharmacol. 2018; 28:980–2.

45. Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci. 2012; 367:2475–84.

46. Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011; 16:1068–70.

47. Hasselmann HW. Ketamine as antidepressant? Current state and future perspectives. Curr Neuropharmacol. 2014; 12:57–70.

48. Dave NM. Premedication and induction of anaesthesia in paediatric patients. Indian J Anaesth. 2019; 63:713–20.

49. Stoelting RK, Hillier SC. Nonbarbiturate Intravenous Anaesthetic Drugs. In: Pharmacology and Physiology in Anaesthetic Practice. 4th ed. Philadelphia: Lippincott Williams and Wilkin;2006. p. 155–78.
50. Poonai N, Canton K, Ali S, Hendrikx S, Shah A, Miller M, et al. Intranasal ketamine for procedural sedation and analgesia in children: a systematic review. PLoS One. 2017; 12:e0173253.

51. Gyanesh P, Haldar R, Srivastava D, Agrawal PM, Tiwari AK, Singh PK. Comparison between intranasal dexmedetomidine and intranasal ketamine as premedication for procedural sedation in children undergoing MRI: A double-blind, randomized, placebo-controlled trial. J Anesth. 2014; 28:12–8.

52. Khutia SK, Mandal MC, Das S, Basu SR. Intravenous infusion of ketamine-propofol can be an alternative to intravenous infusion of fentanyl-propofol for deep sedation and analgesia in paediatric patients undergoing emergency short surgical procedures. Indian J Anaesth. 2012; 56:145–50.

53. Khajavi M, Emami A, Etezadi F, Safari S, Sharifi A, Shariat Moharari R. Conscious sedation and analgesia in colonoscopy: Ketamine/propofol combination has superior patient satisfaction versus fentanyl/propofol. Anesth Pain Med. 2013; 3:208–13.

54. Atashkhoyi S, Negargar S, Hatami-Marandi P. Effects of the addition of low-dose ketamine to propofol-fentanyl anaesthesia during diagnostic gynaecological laparoscopy. Eur J Obstet Gynecol Reprod Biol. 2013; 170:247–50.

55. Schnabel A, Poepping DM, Kranke P, Zahn PK, Pogatzki-Zahn EM. Efficacy and adverse effects of ketamine as an additive for paediatric caudal anaesthesia: a quantitative systematic review of randomized controlled trials. Br J Anaesth. 2011; 107:601–11.
56. Dahmani S, Michelet D, Abback PS, Wood C, Brasher C, Nivoche Y, et al. Ketamine for perioperative pain management in children: a meta-analysis of published studies. Paediatr Anaesth. 2011; 21:636–52.

57. Aliena SP, Lini C, Chirayath JJ. Comparison of postoperative analgesic effect of caudal bupivacaine with and without ketamine in pediatric subumbilical surgeries. J Anaesthesiol Clin Pharmacol. 2018; 34:324–7.
58. Tobias JD. Dexmedetomidine and ketamine: an effective alternative for procedural sedation? Pediatr Crit Care Med. 2012; 13:423–7.
59. Char D, Drover DR, Motonaga KS, Gupta S, Miyake CY, Dubin AM, et al. The effects of ketamine on dexmedetomidine-induced electrophysiologic changes in children. Paediatr Anaesth. 2013; 23:898–905.

60. Ibrahem Amin OA, Kamel AA. Effects of adding ketamine to dexmedetomidine on smooth recovery from isoflurane anesthesia in adults undergoing endoscopic sinus surgery. Egypt J Anaesth. 2020; 36:50–5.

61. Sinha SK, Joshiraj B, Chaudhary L, Hayaran N, Kaur M, Jain A. A comparison of dexmedetomidine plus ketamine combination with dexmedetomidine alone for awake fiberoptic nasotracheal intubation: a randomized controlled study. J Anaesthesiol Clin Pharmacol. 2014; 30:514–9.

62. Kim JG, Lee HB, Jeon SB. Combination of dexmedetomidine and ketamine for magnetic resonance imaging sedation. Front Neurol. 2019; 10:416.

63. Chun EH, Han MJ, Baik HJ, Park HS, Chung RK, Han JI, et al. Dexmedetomidine-ketamine versus dexmedetomidine-midazolam-fentanyl for monitored anesthesia care during chemoport insertion: a prospective randomized study. BMC Anesthesiol. 2016; 16:49.

64. Qiao H, Xie Z, Jia J. Pediatric premedication: a double-blind randomized trial of dexmedetomidine or ketamine alone versus a combination of dexmedetomidine and ketamine. BMC Anesthesiol. 2017; 17:158.

65. Groth C, Connor K, Kaukeinen K, Acquisto N, Chui SH, Cucci M, et al. Multicentric retrospective review of ketamine use in the intensive care unit (ketamine-ICU study). Crit Care Med. 2020; 48:459.
66. Umunna BP, Tekwani K, Barounis D, Kettaneh N, Kulstad E. Ketamine for continuous sedation of mechanically ventilated patients. J Emerg Trauma Shock. 2015; 8:11–5.

67. Yoon SH. Concerns of the anesthesiologist: anesthetic induction in severe sepsis or septic shock patients. Korean J Anesthesiol. 2012; 63:3–10.

68. Wong A, Benedict NJ, Armahizer MJ, Kane-Gill SL. Evaluation of adjunctive ketamine to benzodiazepines for management of alcohol withdrawal syndrome. Ann Pharmacother. 2015; 49:14–9.

69. Pizon AF, Lynch MJ, Benedict NJ, Yanta JH, Frisch A, Menke NB, et al. Adjunct ketamine use in the management of severe ethanol withdrawal. Crit Care Med. 2018; 46:e768–71.

70. Sabharwal V, Ramsay E, Martinez R, Shumate R, Khan F, Dave H, et al. Propofol-ketamine combination therapy for effective control of super-refractory status epilepticus. Epilepsy Behav. 2015; 52:264–6.

71. Ferlisi M, Hocker S, Grade M, Trinka E, Shorvon S. Preliminary results of the global audit of treatment of refractory status epilepticus. Epilepsy Behav. 2015; 49:318–24.

72. Rosati A, De Masi S, Guerrini R. Ketamine for refractory status epilepticus: a systematic review. CNS Drugs. 2018; 32:997–1009.

73. Fujikawa DG. Starting ketamine for neuroprotection earlier than its current use as an anesthetic/antiepileptic drug late in refractory status epilepticus. Epilepsia. 2019; 60:373–80.

74. Ilvento L, Rosati A, Marini C, L'Erario M, Mirabile L, Guerrini R. Ketamine in refractory convulsive status epilepticus in children avoids endotracheal intubation. Epilepsy Behav. 2015; 49:343–6.

75. Shrestha GS, Joshi P, Chhetri S, Karn R, Acharya SP. Intravenous ketamine for treatment of super-refractory convulsive status epilepticus with septic shock: a report of two cases. Indian J Crit Care Med. 2015; 19:283–5.

76. Mogahd MM, Mahran MS, Elbaradi GF. Safety and efficacy of ketamine-dexmedetomidine versus ketamine-propofol combinations for sedation in patients after coronary artery bypass graft surgery. Ann Card Anaesth. 2017; 20:182–7.

77. Mazzeffi M, Johnson K, Paciullo C. Ketamine in adult cardiac surgery and the cardiac surgery Intensive Care Unit: An evidence-based clinical review. Ann Card Anaesth. 2015; 18:202–9.

78. Hudetz JA, Patterson KM, Iqbal Z, Gandhi SD, Byrne AJ, Hudetz AG, et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2009; 23:651–7.

79. Kranaster L, Kammerer-Ciernioch J, Hoyer C, Sartorius A. Clinically favourable effects of ketamine as an anaesthetic for electroconvulsive therapy: a retrospective study. Eur Arch Psychiatry Clin Neurosci. 2011; 261:575–82.

80. Firouzian A, Tabassomi F. Is ketamine-propofol mixture (ketofol) an appropriate alternative induction agent for electroconvulsive therapy? Saudi J Anaesth. 2013; 7:476–7.

81. Yalcin S, Aydoğan H, Selek S, Kucuk A, Yuce HH, Karababa F, et al. Ketofol in electroconvulsive therapy anesthesia: two stones for one bird. J Anesth. 2012; 26:562–7.

82. Shams T, El-Masry R. Ketofol-dexmedetomidine combination in ECT: a punch for depression and agitation. Indian J Anaesth. 2014; 58:275–80.

83. Kellner CH, Iosifescu DV. Ketamine and ECT: better alone than together? Lancet Psychiatry. 2017; 4:348–9.

84. Schwartz J, Murrough JW, Iosifescu DV. Ketamine for treatment-resistant depression: recent developments and clinical applications. Evid Based Ment Health. 2016; 19:35–8.
85. Aigbedia SO, Tobi KU, Amadasun FE. A comparative study of ketamine gargle and lidocaine jelly application for the prevention of postoperative throat pain following general anaesthesia with endotracheal intubation. Niger J Clin Pract. 2017; 20:677–85.

86. Rudra A, Ray S, Chatterjee S, Ahmed A, Ghosh S. Gargling with ketamine attenuates the postoperative sore throat. Indian J Anaesth. 2009; 53:40–3.
87. Chan L, Lee ML, Lo YL. Postoperative sore throat and ketamine gargle. Br J Anaesth. 2010; 105:97.

88. Ahuja V, Mitra S, Sarna R. Nebulized ketamine decreases incidence and severity of post-operative sore throat. Indian J Anaesth. 2015; 59:37–42.

89. Sener S, Eken C, Schultz CH, Serinken M, Ozsarac M. Ketamine with and without midazolam for emergency department sedation in adults: a randomized controlled trial. Ann Emerg Med. 2011; 57:109–14.

90. Lohit K, Srinivas V, Kulkarni C. Shaheen. A clinical evaluation of the effects of administration of midazolam on ketamine-induced emergence phenomenon. J Clin Diagn Res. 2011; 5:320–3.
91. Akhlaghi N, Payandemehr P, Yaseri M, Akhlaghi AA, Abdolrazaghnejad A. Premedication with midazolam or haloperidol to prevent recovery agitation in adults undergoing procedural sedation with ketamine: a randomized double-blind clinical trial. Ann Emerg Med. 2019; 73:462–9.

92. Li CC, Wu ST, Cha TL, Sun GH, Yu DS, Meng E. A survey for ketamine abuse and its relation to the lower urinary tract symptoms in Taiwan. Sci Rep. 2019; 9:7240.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download