Abstract
Background
Protection of healthcare providers (HCP) has been a serious challenge in the management of patients during the coronavirus 2019 (COVID-19) pandemic. Additional physical barriers have been created to enhance personal protective equipment (PPE). In this study, user acceptability of two novel barriers was evaluated and the performance of airway management using PPE alone versus PPE plus the additional barrier were compared.
Methods
An open-label, double-armed simulation pilot study was conducted. Each participant performed bag-mask ventilation and endotracheal intubation using a GlideScope in two scenarios: 1) PPE donned, followed by 2) PPE donned plus the addition of either the isolation chamber (IC) or aerosol box (AB). Endotracheal intubation using videolaryngoscopy was timed. Participants completed pre- and post-simulation questionnaires.
Results
Twenty-nine participants from the Department of Anesthesia were included in the study. Pre- and post-simulation questionnaire responses supported the acceptance of additional barriers. There was no significant difference in intubating times across all groups (PPE vs. IC 95% CI, 26.3, 35.1; PPE vs. AB 95% CI, 25.9, 35.5; IC vs. AB 95% CI, 23.6, 39.1). Comparison of post-simulation questionnaire responses between IC and AB showed no significant difference. Participants did not find the additional barriers negatively affected communication, visualization, or maneuverability.
Protecting healthcare providers (HCP) from infection has been a highly prioritized goal during the management of the coronavirus 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. While respiratory droplets and contact transmission are recognized as the most important routes of transmission for SARS-CoV-2, airborne dissemination of the virus may occur in settings where aerosol generating procedures (AGPs) are performed [2,3]. It is recommended that HCP use airborne, droplet and contact precautions personal protective equipment (PPE) during AGPs performed on suspected or confirmed COVID-19 patients [4–6].
Inadequate supply of appropriate PPE has been an ongoing worldwide concern, and unacceptably high rates of HCP infection and deaths as a consequence of COVID-19 nosocomial spread have been partially attributed to the shortage of PPE [7–9]. In addition, recent simulation studies looking at the protection of HCP wearing properly donned PPE found droplet markers on exposed neck, ears, hair, and shoes, suggesting that even with available standard recommended PPE there is still a potential risk of safety breaches and contamination [10,11]. A recent comprehensive Cochrane review summarizes the published evidence on PPE preventing infectious disease contamination of HCP, and overall found limited certainty in the evidence due to the limited, low powered studies [12]. This review stated that covering more exposed body surfaces of HCP can lead to better protection but often with more difficulty with donning or doffing, and less user comfort that can both lead to greater HCP contamination [12]. As a response to the inadequate PPE supply and concern for contamination, HCP have considered the use of innovative barrier enclosures during AGPs as supplemental protection against aerosol and droplet exposure [13–18].
There is wide recognition that acceptability should be examined when new interventions in health care are designed and implemented [19]. Both patient and provider acceptance of interventions have been studied, and it has been observed that the degree of acceptability can alter the effectiveness and uptake of the intervention [19–22]. During the COVID-19 pandemic, one of the first protective barriers proposed was an acrylic aerosol box (AB) covering a patient’s head [13]. However, the use of a rigid box has been reported to restrict arm movement [14]. Variations on the original AB design, and other designs that create isolation chambers (ICs) by utilizing a polyvinyl chloride plastic (PVC) rigid frame and a clear plastic bag, have subsequently been described [15–17]. However, no studies comparing acceptability of these devices have been reported.
In this pilot study, we tested two of the novel barrier enclosures, the AB and IC, in a simulated environment. The primary objective was to assess the acceptability of these additional physical barriers during a simulated airway management scenario performed by anesthesia providers. Secondary objectives included comparing the performance of airway management using PPE alone versus PPE plus the additional barrier, comparing the IC to the AB and observing potential limitations of the additional barriers. We hypothesize that the addition of a patient barrier will be acceptable to anesthesia providers without negatively impacting the provider’s ability to perform the simulated airway management procedures.
After Research Ethics Board (REB) approval (Human Research Ethics — Western University HSREB 115895, approved May 1, 2020), an open-label, double-armed pilot study was conducted in May 2020 at London Health Sciences Centre (LHSC), a tertiary care center in London, Canada. Study participants were voluntarily recruited from the Department of Anesthesia & Perioperative Medicine at Western University through a department-wide email invitation. Any resident, fellow, or consultant within the Department of Anesthesia & Perioperative Medicine at Western University satisfied the inclusion criteria. Since this was a pilot study, a sample size of 24 — 12 participants in each group was sought [23,24]. The simulation scenario was setup in an operating room at LHSC. Participation occurred during regular clinical shifts at LHSC so neither additional risk of COVID-19 hospital exposure nor any increased use of PPE was incurred by the study. Participants received a letter of information that was reviewed with a study coordinator prior to the simulation, and written informed consent was obtained.
Two physical barriers were assessed — the PVC rigid frame IC covered by a clear plastic bag proposed by Cubillos et al. [17], and a polycarbonate AB (Supplementary Fig. 1). Participants were divided into two groups by alternating assignment on arrival to the simulation. Group 1 was assigned to use the IC and Group 2 was assigned to use the AB. Both barriers were pre-constructed and ready for use by the participant. One of the study coordinators cut vertical armholes in the plastic bag of the IC at approximately mid-abdominal level and shoulder-width apart prior to the simulation session.
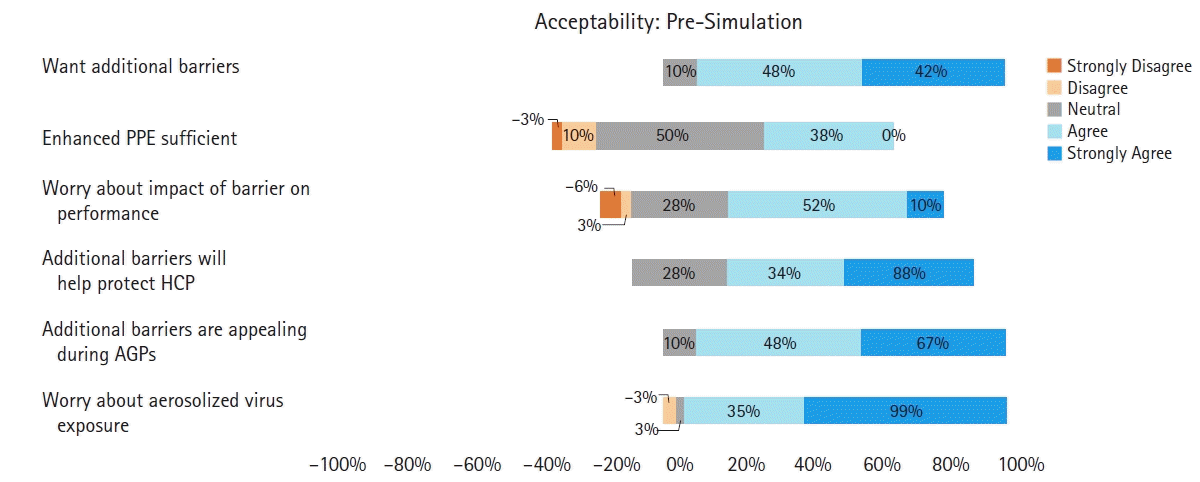
A pre-simulation questionnaire was completed by all participants (Table 1). In an effort to preserve supplies of PPE, participants were required to wear limited PPE. As a minimum, limited PPE required a mask (surgical mask or N95 respirator) and eye-shield, as these items were anticipated to most interfere with the use of additional patient barriers in terms of communication and vision, respectively.
Pre-simulation Questionnaires and Median Responses
During simulation, all participants, wearing limited PPE first performed bag-mask ventilation followed by endotracheal intubation using a GlideScope (Verathon, USA) with a size 3 blade and a 7.5 or 8.0 endotracheal tube with a stylet on a mannequin head (Airsim Advance X by TruCorp., Ireland). Immediately after, all participants wearing the same limited PPE performed the same airway management procedures using their assigned additional patient barrier. During both scenarios, the participant was allowed to make ergonomic and equipment adjustments according to personal preference such as bending of the styleted endotracheal tube, positioning of the equipment, and changes in the height of the bed. No verbal cues were given to the participants.
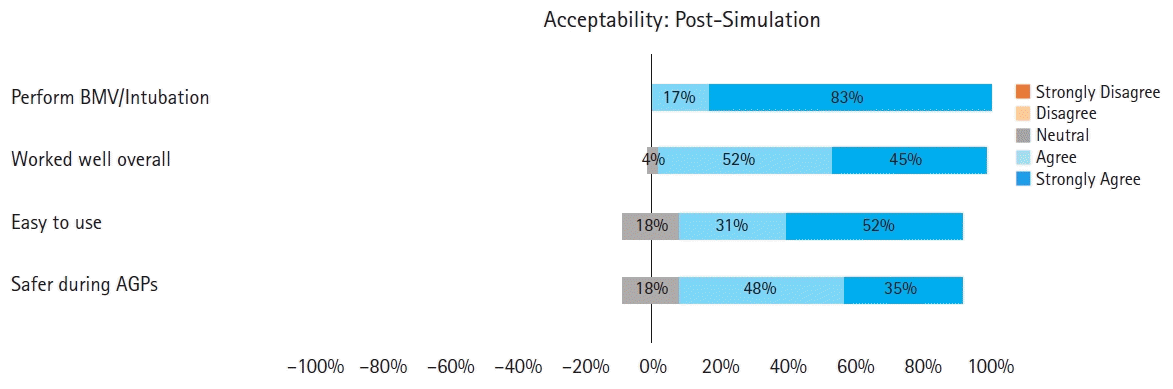
Time to endotracheal intubation was recorded for all simulations. This was standardized to begin when the participant picked up the GlideScope blade and concluded when the endotracheal cuff was inflated. All participants received expert assistance for airway management — either one of the study coordinators or another study participant. Following the simulated activities, the participant completed a post-simulation questionnaire (Table 2).
Post-simulation Questionnaire and Median Responses
Both questionnaires consisted of seven five-point Likert-scale questions, in addition to one binomial question (question 1) in the pre-simulation questionnaire, created for this pilot study to address the proposed objectives (Tables 1 and 2) [25]. The primary outcome of acceptability was assessed by evaluating the five-point Likert scale median and interquartile range (Q1, Q3) of the questions assessing acceptability, where a response of 3.5 or greater was considered positive and 2 or less considered negative [26,27]. Intubating times for each group were recorded. Participants identified whether they were assigned to the IC or AB on the post-simulation questionnaire to facilitate comparison of the IC to the AB. Limitations of the additional barriers were assessed through post-simulation questionnaire responses. All questionnaires were completed anonymously.
Statistical analysis comparing intubating times between PPE alone and IC, and PPE alone and AB was completed using a paired t-test. Intubating times and post-simulation questionnaire results between IC and AB were compared using a non-paired t-test. A 95% CI was used, and P value < 0.05 was considered significant.
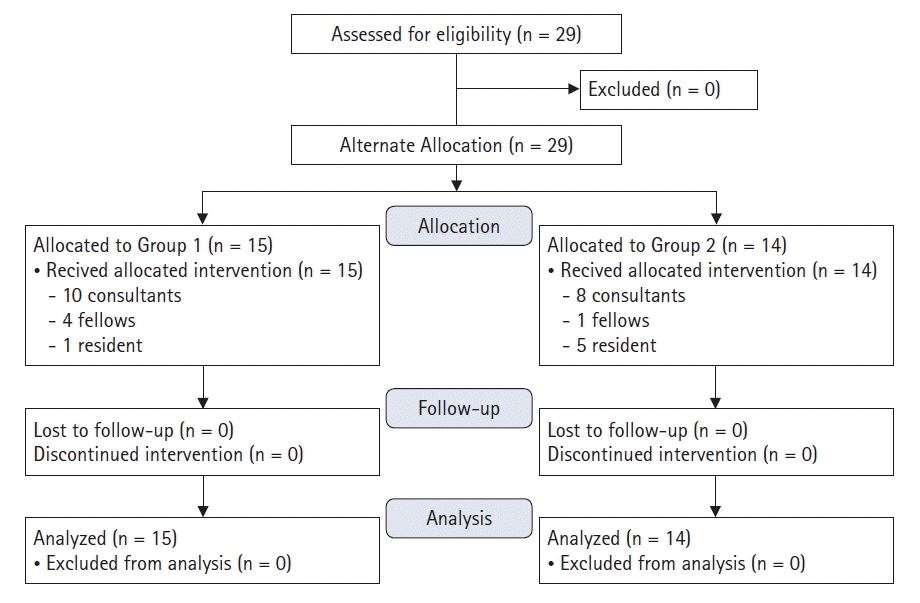
A total of 29 participants from the Department of Anesthesia & Perioperative Medicine were included in the pilot study (Fig. 1). On simulation day 3, the threshold of 24 participants was surpassed and the study ended.
For the questionnaires, responses to the one binomial pre-simulation question (question 1), and to the seven five-point Likert questions in the pre- and post-simulation questionnaire were evaluated and the median (Q1, Q3) values were calculated. A summary of the pre- and post-simulation questionnaire responses can be found in Tables 1 and 2, respectively. Regarding the primary outcome of acceptability, median (Q1, Q3) values for the pre- and post-simulation questions assessing acceptability to anesthesia providers were all positive (> 3.5), indicating acceptability of the additional barriers (Figs. 2 and 3, respectively).
Intubating times were assessed for limited PPE (30.1 ± 11.7 s), Group 1 IC (31.4 ± 20.1 s), and Group 2 AB (31.3 ± 23.3 s). Intubating times for PPE to IC and PPE to AB (95% CI, 26.3, 35.1, P = 0.752; 95% CI, 25.9, 35.5, P = 0.824; respectively) and IC to AB (95% CI, 23.6, 39.1, P = 0.995) were compared and there was no statistically significant difference between any of the groups.
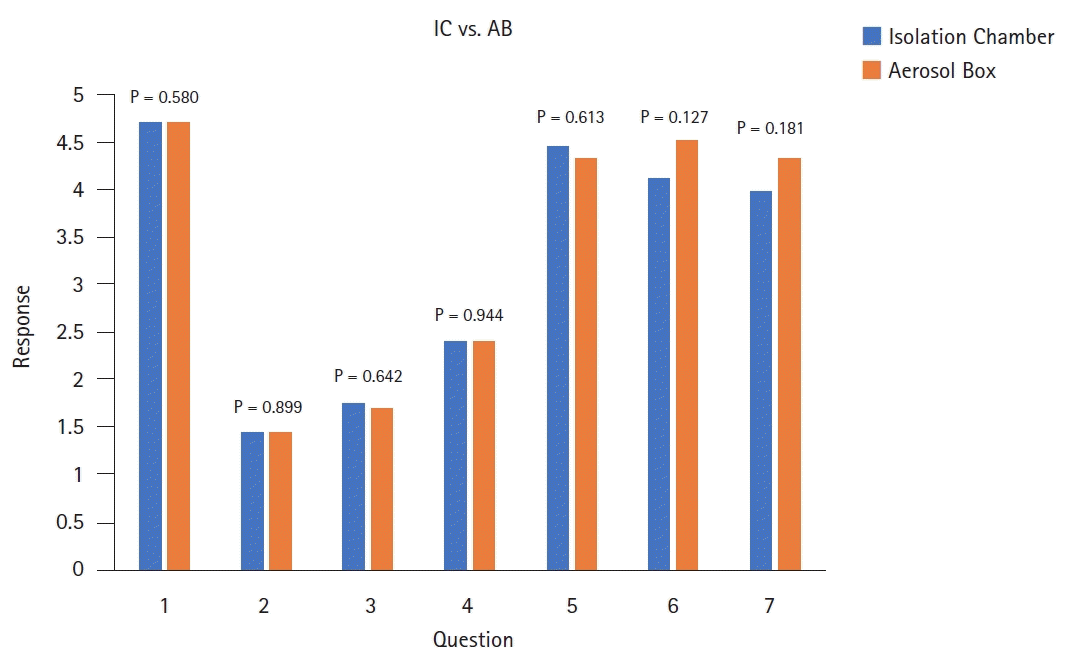
Post-simulation IC and AB questionnaire responses to assess the primary outcome demonstrated no significant difference between IC and AB for all questionnaire responses (questions 1–7: P = 0.580, P = 0.899, P = 0.642, P = 0.944, P = 0.613, P = 0.127, P = 0.181, respectively) (Fig. 4).
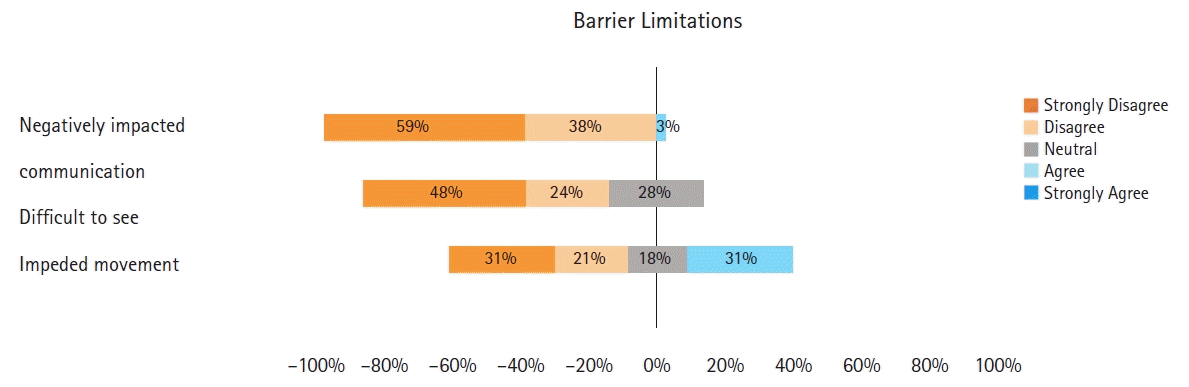
Post-simulation responses regarding communication, vision, and movement indicated the participants did not find the additional barriers limiting (median [Q1, Q3] responses 1 [1, 2], 1.5 [1, 3] and 2 [1, 4], respectively) (Fig. 5).
As much is still being learned about COVID-19, several key points suggest the importance of enhanced barrier protection. Viral particles have been found on surfaces and in the air up to 4 m from patients, and viable on surfaces for up to 72 h and as aerosols for at least 3 h [3,28]. Studies investigating respiratory and cough particles have shown contamination of HCP wearing standard PPE, while an AB or plastic drape reduced macroscopic contamination of the HCP and the environment [11,14,18].
Our data suggests additional patient barriers are acceptable to anesthesia providers during simulated bag-mask ventilation and endotracheal intubation using videolaryngoscopy. The majority of participants in this study had not used an additional patient barrier prior to the simulation, but stated they worry about aerosolized viral particles and found additional patient barriers to be appealing. While studies have described limited arm movement with the AB design [14,15], Cubillos et al. [17] did not report restrictions of movement with the IC design. Overall, participant responses indicated that the additional barriers were easy to use, allowed for all simulated airway management maneuvers to be executed, and had the potential to complement the current PPE clinical safety measures.
The results in this study show a high acceptance rate of additional barriers to protect anesthesia providers and indicate that implementation would likely be effective with a high user uptake. This data is translatable to other anesthesia providers, although situational variations encountered in daily practice can limit widespread applicability in all circumstances. The additional patient barriers also have the potential for use in a variety of other clinical settings where risky AGPs are performed. The absence of observed limitations to using a barrier in this study may not be applicable to all other scenarios.
Limitations of this study include the physical, psychological, and semantic realism of the simulation environment that differ significantly from the real clinical environment where challenging circumstances such as a potentially difficult airway can significantly affect airway management outcomes and overall acceptability of the barriers [29]. Additionally, the results cannot be extrapolated to conclude that similar results would be obtained during airway management using different equipment, such as direct laryngoscopy or a supraglottic device. There was no randomization in this study, as participants were alternately allocated upon arrival to the simulation, although their arrival was not planned or pre-arranged. A selection bias might result from having volunteers. Finally, all participants performed the AGPs with PPE alone prior to the additional barrier and may have become more familiar with the mannequin airway.
Further research into the additional patient barriers is warranted before they can be safely recommended for clinical use. Barriers may inadvertently create a false sense of security causing more harm than benefit. Additionally, prior to the use of any additional patient barrier, HCP should receive proper orientation and simulation-based training in order to optimally benefit from the device. However, there is no evidence that novel barrier devices are associated with less viral transmission. Each barrier, although intuitively appealing, could have unintended consequences such as infection transmission caused by PPE breaches or inadequate doffing and cleaning between uses. Quantitative studies comparing barriers, and examining their enhanced level of protection are pertinent and needed. Moreover, investigating other barrier modifications, such as the application of continuous suction to create a negative pressure environment and flow to further enhance the safety of the environment, is essential. In the long-term, it will also be crucial to monitor any meaningful and significant difference in the rate of nosocomial spread with the addition of patient barriers.
Overall, with the worldwide crisis generated by this pandemic that significantly impacts HCP safety and health care system stability, we have learned that further study is warranted when new tools are introduced as potential devices that might impact critical outcomes. This novel study is the first to assess the acceptability of additional patient barriers, and to further compare two differently designed devices. Our pilot data suggests that anesthesia providers positively accept the use of additional patient barriers during AGP and there would be support to have these barriers as an option to complement the standard PPE recommendations.
ACKNOWLEDGMENTS
Dr. Herman Sehmbi for review and comments on the study, Dr. Mahesh Nagappa for review and comments on the study design, and Dr. Phil Jones for review and feedback on the manuscript.
Notes
Author Contributions
Jill Querney (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing – original draft; Writing – review & editing)
Javier Cubillos (Conceptualization; Data curation; Methodology; Writing – review & editing)
Youshan Ding (Resources; Writing – review & editing)
Richard Cherry (Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Writing – review & editing)
Kevin Armstrong (Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Writing – review & editing)
Supplementary Materials
Supplementary Fig. 1.
Images of the additional barriers used in the simulation. (A) Picture of the IC, (B) Picture of the AB. IC: isolation chamber, AB: aerosol box.
References
1. Adalja AA, Toner E, Inglesby TV. Priorities for the US health community responding to COVID-19. JAMA. 2020; 323:1343–4.

2. World Health Organization. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations [Internet]. Geneva: WHO;2020. Mar 29 [cited 2020 Aug 17]. Available from https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
3. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020; 382:1564–7.

4. World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages: interim guidance [Internet]. Geneva: WHO;2020. Apr 6 [cited 2020 Aug 17]. Available from https://apps.who.int/iris/handle/10665/331695.
5. Public Health Agency of Canada. Infection prevention and control for COVID-19: second interim guidance for acute healthcare settings [Internet]. Ottawa: PHAC;2020. May. [updated 2021 Feb 12; cited 2020 Aug 17]. Available from https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/health-professionals/infection-prevention-control-covid-19-second-interim-guidance.html.
6. Cook TM, El-Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020; 75:785–99.
7. Ranney ML, Griffeth V, Jha AK. Critical supply shortages - the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020; 382:e41.

8. Hopman J, Allegranzi B, Mehtar S. Managing COVID-19 in low- and middle-income countries. JAMA. 2020; 323:1549–50.

9. Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic. JAMA. 2020; 323:1912–4.

10. Weissman DN, de Perio MA, Radonovich LJ Jr. COVID-19 and risks posed to personnel during endotracheal intubation. JAMA. 2020; 323:2027–8.

11. Feldman O, Meir M, Shavit D, Idelman R, Shavit I. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. JAMA. 2020; 323:2091–3.

12. Verbeek JH, Rajamaki B, Ijaz S, Sauni R, Toomey E, Blackwood B, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2020; 4:CD011621.

13. Everington K. Taiwanese doctor invents device to protect US doctors against coronavirus [Internet]. Taipei: Taiwan News. 2020 May 23. Available from https://www.taiwannews.com.tw/en/news/3902435.
14. Canelli R, Connor CW, Gonzalez M, Nozari A, Ortega R. Barrier enclosure during endotracheal intubation. N Engl J Med. 2020; 382:1957–8.

15. Begley JL, Lavery KE, Nickson CP, Brewster DJ. The aerosol box for intubation in coronavirus disease 2019 patients: an in-situ simulation crossover study. Anaesthesia. 2020; 75:1014–21.

16. Malik JS, Jenner C, Ward PA. Maximising application of the aerosol box in protecting healthcare workers during the COVID-19 pandemic. Anaesthesia. 2020; 75:974–5.

17. Cubillos J, Querney J, Rankin A, Moore J, Armstrong K. A multipurpose portable negative air flow isolation chamber for aerosol-generating procedures during the COVID-19 pandemic. Br J Anaesth. 2020; 125:e179–81.

18. Matava CT, Yu J, Denning S. Clear plastic drapes may be effective at limiting aerosolization and droplet spray during extubation: implications for COVID-19. Can J Anaesth. 2020; 67:902–4.

19. Sekhon M, Cartwright M, Francis JJ. Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res. 2017; 17:88.

20. Diepeveen S, Ling T, Suhrcke M, Roland M, Marteau TM. Public acceptability of government intervention to change health-related behaviours: a systematic review and narrative synthesis. BMC Public Health. 2013; 13:756.

21. Ayala GX, Elder JP. Qualitative methods to ensure acceptability of behavioral and social interventions to the target population. J Public Health Dent. 2011; 71 Suppl 1:S69–79.

22. Ndejjo R, Musinguzi G, Nuwaha F, Wanyenze RK, Bastiaens H. Acceptability of a community cardiovascular disease prevention programme in Mukono and Buikwe districts in Uganda: a qualitative study. BMC Public Health. 2020; 20:75.

23. Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Stat. 2005; 4:287–91.

24. Moore CG, Carter RE, Nietert PJ, Stewart PW. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci. 2011; 4:332–7.

25. Krosnick JA, Presser S. Question and questionnaire design. 2nd ed. Marsden PV, Wright JD, editors. West Yorkshire: IEmerald Group publishing limited;2010. p. 263–313.
26. Sullivan GM, Artino AR Jr. Analyzing and interpreting data from Likert-type scales. J Grad Med Educ. 2013; 5:541–2.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download