This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Pierre Robin sequence (PRS) patients have an increased risk of difficult intubation due to anatomical airway abnormalities, and intubation simulation with a three-dimensional (3D) printed airway model before anesthesia may facilitate safe airway management.
Case
We describe the case of a 6.5-year-old boy with a history of PRS (a triad of micrognathia, glossoptosis, and airway obstruction), tracheostomy, and subglottic fibrosis who required general anesthesia. Preparation for this potentially difficult intubation included estimation of endotracheal tube size using a 3D printed airway model derived from 3D computed tomography of the airway, which enabled successful endotracheal intubation via video laryngoscopy.
Conclusions
If general anesthesia is necessary in patients with dysmorphic features such as PRS and there is a history of tracheal pathology, the possibility of difficult intubation should always be considered and simulation of endotracheal intubation using a 3D printed model of the airway can be helpful clinically in such situations.
Go to :

Keywords: Airway management, Computer simulation, Endotracheal intubation, Pierre Robin sequence, Pierre-Robin syndrome, 3D printing
Pierre Robin sequence (PRS) is a clinical disease involving the triad of micrognathia, glossoptosis, and airway obstruction [
1]. Patients with PRS may require general anesthesia for various reasons and the clinical triad of PRS can present the anesthetist with substantial challenges, including airway obstruction and difficult intubation [
1]. The treatment of patients with a history of tracheostomy can be complicated by problems such as cicatrization of the airway mucosa and tracheal stenosis [
2], and they can require airway evaluation via radiological imaging as well as physical examination prior to general anesthesia with endotracheal intubation. There has been no report of using three-dimensional (3D) airway modeling via 3D computed tomography (CT) in the present PRS patient with a history of tracheostomy. We describe the case report of a successful endotracheal intubation using video laryngoscopy and 3D printed airway model.
Case Report
Written informed consent was obtained from the parent. The patient was a 6.5-year-old boy with PRS (height 110 cm, weight 15.46 kg) who was admitted due to persistent leakage at the closure site after percutaneous endoscopic gastrostomy removal. He had a history of failed intubation and emergent tracheostomy at birth due to severe micrognathia and glossoptosis. Granular tissue removal at the tracheostomy site was performed due to subglottic fibrosis four months after tracheostomy. Micrognathia and repaired cleft palate were identified by physical examination.
We decided to use 3D printed airway modeling, because there was a possibility of difficult intubation and it was difficult to predict the appropriate endotracheal tube size. The open-source program InVersalius (InVersalius 3.0, Renato Archer Information Technology Center, Brazil) was used for 3D printed airway modeling and for the generation of .stl files, and the 3D airway model was printed using CreatBot (CreatBot F430, Henan Suwei Electronic Technology Co., Ltd., China). The 3D printed airway model did not reveal airway narrowing (
Fig. 1A), and simulation of endotracheal intubation was successful with an internal diameter (ID) 5.5 sized cuffed endotracheal tube (Hi-contour oral cuffed tracheal tube, Covidien, USA) (
Fig. 1B).
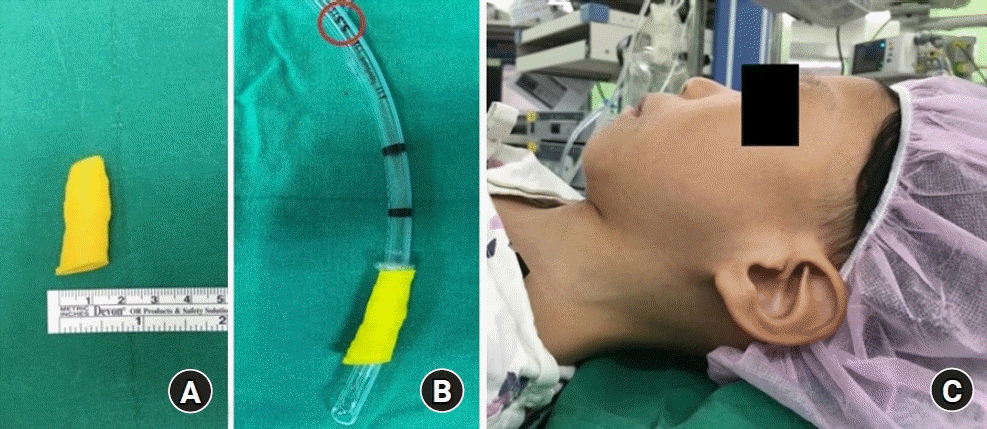 | Fig. 1.Printed three-dimensional (3D) airway model and patient’s dysmorphic face. (A) Printed 3D airway model derived from 3D chest airway computed tomography. (B) Simulation of intubation with a 3D printed airway model. Simulated endotracheal intubation was successful with an internal diameter 5.5 sized cuffed endotracheal tube. (C) The patient’s dysmorphic facial features. He had a small chin, mild retrognathia, and a short thyromental distance. 
|
The patient had a small chin and his range of movement from ear to orbit was estimated to be over 120 degrees (
Fig. 1C). General anesthesia induction and intubation were planned with reference to the difficult airway algorithm described in the 2013 American Society of Anesthesiologists guidelines [
3]. Before the induction of anesthesia, ID 4.5, 5.0, 5.5, and 6.0 cuffed and uncuffed endotracheal tubes were secured, and pre-emptive preparations were made for laryngeal mask airway, video laryngoscope (KoMAC video laryngoscope, KoMAC Co., Ltd., Korea), stylet, nasopharyngeal airway, oropharyngeal airway, and fiberoptic bronchoscope. Mask ventilation was possible after the administration of intravenous thiopental 75 mg, followed by rocuronium 10 mg. Successful endotracheal intubation was performed via video laryngoscopy with a #2 blade, a stylet, and an ID 5.5 cuffed endotracheal tube. Ventilation was performed after confirming the suitability of the endotracheal tube using a leakage test. There were no subsequent complications, and he was discharged on postoperative day 6.
Go to :

Discussion
Two aspects of the current patient’s pre-anesthesia plan were focused on: the potential for difficult airway intubation and the difficulty of determining the appropriate endotracheal tube size due to his history of tracheostomy and subglottic fibrosis. Predictors of difficult intubation in congenital syndromes include the presence of dysmorphic features, limited neck, limited mouth opening, restricted mobility of temporo-mandibular joints, a large tongue, limited submandibular space, and the presence of structural abnormalities in the laryngo-tracheal passage [
4]. All patients with PRS have airway obstruction, as it is a requirement for the clinical diagnosis [
1]. Although airway obstruction tends to improve with age, the very difficult intubation with direct laryngoscopy should be always considered in the pre-anesthesia plan [
5]. In the patients with PRS, fiberoptic intubation is the gold standard, but numerous other approaches have been used successfully including direct video laryngoscopy and fiberoptic intubation via laryngeal mask airway [
4]. Successful intubation was performed on the first attempt using video laryngoscopy in the present case.
An important consideration during the pre-anesthesia planning in the present case was the undesirability of using the age-dependent formula to determine endotracheal tube size because the patient had a history of tracheostomy. Many reports have described methods for measuring airway size via imaging modalities such as X-ray, CT, and ultrasonography, but two-dimensional imaging methods have inherent limitations [
6–
8]. X-ray tends to overestimates of tracheal diameter [
9], and high-quality laryngeal images by CT are not routinely warranted because images by CT can be affected by airway size, reconstruction algorithm, composition of the airway phantom, and CT scanner types [
10]. The quality of ultrasonography also depends on the ex¬perience of the operator [
11].
Following advances in medical technology, many studies that have successfully implemented two-dimensional radiographic images in three dimensions have been reported in the surgical field [
12,
13]. Various attempts to use 3D models have been made in the field of anesthesia, but there have been only a few resulting reports and the reality is that those attempts were focused on education rather than actual clinical application [
14].
In the current case, a 3D printed airway model was successfully used to confirm that there were no anatomical abnormalities of the airway. It was also possible to predict the appropriate endotracheal tube size. This indicates that simulation of intubation via 3D printed airway modeling before anesthesia can be helpful with regard to safe airway management, and reducing the risks of airway irritation, injury, and edema by reducing the number of endotracheal intubation attempts in cases where difficult intubation is expected due to anatomical abnormalities of the face and airway. Accurate airway evaluation using 3D printing may help anesthesiologists to understand the anatomy of the airways and is useful for developing a better pre-anesthesia plan via simulation of intubation.
However, there are several limitations to the approach. Three-dimensional implementation of medical imaging data requires high-quality imaging, and it is more difficult to apply 3D conversion software programs to airway images than to images of solid organs because of the air layer. In addition, 3D printed airway models may differ from the actual airway because of flexibility.
In conclusion, if general anesthesia including endotracheal intubation is necessary in patients with dysmorphic features such as PRS and there is a history of tracheal pathology, the possibility of difficult intubation should always be considered, and the difficult airway algorithm should be followed. Simulation of endotracheal intubation using a 3D printed model of the airway can be helpful clinically in such situations.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download