Abstract
Background
Magnetic resonance neurography shows the brachial plexus cords in the subcoracoid tunnel beneath the pectoralis minor. With an ultrasound scan along the brachial line, the brachial plexus cords in the subcoracoid tunnel can be targeted using an in-plane needle approach. We describe this new approach to the infraclavicular block called the “subcoracoid tunnel block.”
Case
Twenty patients were administered with the ultrasound-guided subcoracoid tunnel block for the below-elbow surgery. The contact of the needle tip with cords was visible in all 20 patients. With neurostimulation, the posterior cord was identified in 11 (55%) and medial cord in 9 (45%) patients on the first needle pass. The subcoracoid tunnel block was successful in 16 patients (80%).
The ultrasound-guided infraclavicular block (ICB) has several potential advantages for a single shot and continuous block of the brachial plexus [1,2]. Currently, the techniques for ICB include the lateral parasagittal, costoclavicular, and retroclavicular in-plane approaches [3–5]. Out of these, the lateral parasagittal approach in which the needle tip is placed posterior to the artery is most widely practiced. The injection of local anesthetics (LAs) posterior to the axillary artery (AA) produces a U-shape hypoechoic shadow, which has been famously described as a double-bubble sign [6]. Compared to the lateral parasagittal approach, the ultrasound-guided costoclavicular approach produces more rapid and effective anesthesia as the brachial plexus cords are closely clustered in the costoclavicular space [7,8].
Studies using the frontal slab technique of magnetic resonance neurography showed the subcoracoid tunnel beneath the pectoralis minor in an oblique longitudinal plane [9–11]. Based on that finding, we propose a novel approach to ICB, which we describe as the “subcoracoid tunnel block.” In this approach, with the ultrasound scan along the brachial line, the cords of brachial plexus are visualized in the infraclavicular area below the pectoralis minor muscle [12]. In this case series, we employed the subcoracoid tunnel block for 20 patients undergoing below-elbow surgery. Our primary aim was to assess the needle-cord visualization on ultrasound when performing the block. Our secondary aim was to evaluate the identification of cords on neurostimulation, block success rate, and complications if any.
Twenty patients aged 20 to 60 years undergoing below-elbow surgery under the subcoracoid tunnel block were enrolled for this case series from January 2019 to December 2019 after obtaining approval of the hospital's ethical committee (Sancheti Institute of Orthopedics and Rehabilitation, Pune, India). Written informed consent was obtained from all patients. Patients with an American Society of Anesthesiologist physical status greater than III, pregnancy, neuromuscular diseases, skin infections at the needle insertion site, a prior surgery in the infraclavicular fossa, a history of brachial plexus injuries, a bleeding disorder or an allergy to LAs were excluded.
In the supine position, the patient's infraclavicular area was cleaned with an antiseptic solution and draped with sterile sheets; the linear ultrasound probe was wrapped in sterile Tegaderm. Sedation was not induced before or during the block procedure. For the subcoracoid tunnel block, the ultrasound probe was placed along the brachial line formed by joining the external surface landmarks C6 tubercle, mid-clavicular point, and AA [11]. The probe was placed with its proximal end towards the mid-clavicular point and distal end with a marker towards the apex of the axilla (Fig. 1A). The ultrasound scan demonstrated the AA sandwiched between the cords of the brachial plexus in the subcoracoid tunnel. The probe position and needle entry point at the probe's distal end were marked for the in-plane needle approach from a caudal to cephalad direction (Fig. 1B). With a slight lateral or medial tilt of the probe, the cords were seen around the AA.
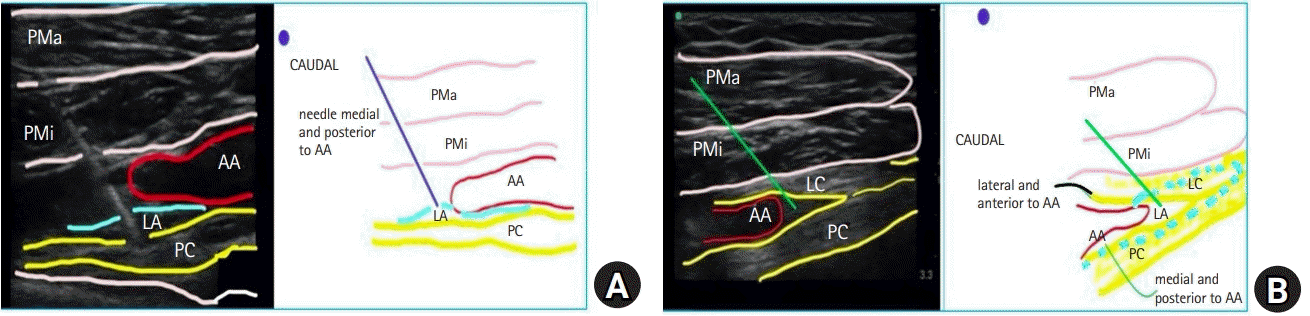
A 100-mm nerve stimulator needle connected to the nerve stimulator was used for the block. A medial tilt demonstrated the posterior (posterior and medial to the AA) and medial (anterior and medial cord to the AA) cords, while a slight lateral tilt of the probe demonstrated the lateral cord (anterior and lateral to the AA). Neurostimulation at 0.4 Ma was applied to identify these cords during the first pass. The probe was tilted medially, and the needle was advanced to position its tip above the posterior or medial cord. After neurostimulation and desired muscle contractions (posterior cord: extension of metacarpophalangeal joints; medial cord: flexion of the metacarpophalangeal joints), the LA was injected (Fig. 2A). The probe was tilted laterally. The needle tip was repositioned above the lateral cord, and the LA was injected (Fig. 2B). A total of 30 ml of 0.5% bupivacaine (25 ml at the posterior or medial cord and 5 ml at the lateral cord) with 1 μg/kg clonidine was injected in 5-ml boluses.
During the block, ultrasound images at the following points were saved: (1) the scan along the brachial line in the oblique longitudinal plane; (2) medial tilt and needle contact with the cord and LA injection; and (3) lateral tilt and needle contact with the cord and LA injection. All images were downloaded on a hard disk in dedicated folders for a later review. For each block, the visibility of contact of the needle tip with the cords on ultrasound images was scored on a 5-point scale; 1: 0–20%, 2: 20–40%, 3: 40–60%, 4: 60–80%, 5: 80–100%.
Patients were assessed at 5-min intervals after the LA injection for the onset of sensory and motor blocks. The sensory block was assessed by loss of pain to a pinprick in the dermatomal areas of the forearm (lateral, medial, and posterior aspects) and the arm (medial and posterior). The motor block was assessed by loss of elbow flexion (musculocutaneous nerve), wrist flexion (median nerve), wrist extension (radial nerve), and flexion of the last two little fingers (ulnar nerve). The onset times for sensory and motor blocks were recorded. The subcoracoid tunnel block was considered successful if there was complete sensory anesthesia of the forearm, no incisional pain, and no need for additional supplementation with intravenous fentanyl, midazolam, or propofol during the surgery. Postoperatively, patients were assessed for the first analgesic request time (visual analogue scale score > 3) and complete motor block recovery. Injection diclofenac 75 mg iv (Dynapar®, Neon, India) was administered for pain relief in the postoperative period. Patients were followed-up for residual neurological deficits, pneumothorax, or infection at the needle insertion site 48 h postoperatively and before discharge.
The demographic and surgical characteristics of the 20 patients undergoing surgical procedures below the elbow under the subcoracoid tunnel block are shown in Table 1. The contact of the needle tip with the cords was visualized in ultrasound images in all 20 patients. On the 5-point scale, the needle visibility was 5 for all patients. During the first pass of the stimulating needle, the posterior cord was identified in 11/20 (55%) patients and medial cord in 9/20 (45%) patients.
The time to complete sensory and motor blocks was 16.9 ± 2.8 and 25.7 ± 2.8 min, respectively. The first analgesic request time was 628.1 ± 128.9 min, and duration of the motor block was 876.9 ± 285.3 min. The subcoracoid tunnel block was successful in 16/20 (80%) patients. One patient had pain at the incision site, and 3 patients complained of mild to moderate pain on the manipulation of fracture fragments. In the patient with incision site pain, the block was supplemented with infiltration of 1% lignocaine (10 ml) along the incision line. The other 3 patients were administered with intermittent boluses of fentanyl 1 μg/kg and midazolam 0.03 mg/kg iv for completion of the surgery. At the follow-up at discharge, no significant complications were seen in any patient.
In our case series, the visibility of contact of the needle tip with the cords in the subcoracoid tunnel block was 5 in all 20 patients (80–100% visibility). On neurostimulation, the posterior cord was identified in 11 (55%) patients and medial cord in 9 (45%) patients. The subcoracoid tunnel block provided effective surgical anesthesia in 16 (80%) patients. The block could be performed in all patients without technical difficulties or complications.
To our knowledge, the subcoracoid tunnel block has not been previously described in the literature. This technique offers several advantages, including good visibility of the neural structures along the length of the brachial plexus. Compared to the traditional lateral parasagittal approach, the needle is better visualized in the subcoracoid tunnel block. During the scan along the brachial line, the AA is seen initially sandwiched between the cords, and a slight medial or lateral tilt leads to the disappearance of the artery bringing the cords in view. This allows safe placement of the needle tip close to the cords. Pneumothorax has been reported in both lateral parasagittal and costoclavicular approaches [13–15]. As the needle in the subcoracoid tunnel block is inserted caudad to cephalad, it is always directed away from the pleura, thus minimizing the chances of pneumothorax. The 2-dimensional spread of LA is also easy to visualize as it is seen hydro-dissecting in a longitudinal axis between the cords of the brachial plexus.
The frontal slab technique of magnetic resonance neurography generates bright images of the brachial plexus in the longitudinal axis [9–11]. Akin to this, we performed ultrasound with a probe below the clavicle along the brachial line. The brachial line (surface landmarks, C6, mid-point of the clavicle, and the AA) coincided with the oblique longitudinal axis of magnetic neurography that identified the brachial plexus in the subcoracoid tunnel [12]. The ultrasound images along the brachial line demonstrated the positions of posterior, medial, and lateral cords as medial and posterior, medial and anterior, and lateral and anterior to the AA, respectively (Figs. 2A and 2B).
The limitation of our study was the small size of the case series. Unlike the traditional lateral parasagittal approach to ICB in which the needle is positioned below the AA, the subcoracoid tunnel block requires a slight medial or lateral tilt of the probe together with the withdrawal and redirection of the needle tip to place it near the cords. Further comparative studies of the subcoracoid tunnel block with traditional lateral parasagittal and costoclavicular approaches are required to evaluate its safety and efficacy.
To conclude, the subcoracoid tunnel block is an easy, safe, and effective alternative approach for ICB. The ultrasound scan along the brachial line below the clavicle aligns the ultrasound beam parallel to the cords of the brachial plexus, generating a longitudinal image of the brachial plexus cords. With the needle inserted in-plane just below the ultrasound probe, the entire needle path and its tip close to the neural targets can be visualized.
Notes
Author Contributions
Sandeep Diwan (Conceptualization; Investigation; Methodology; Project administration; Writing – original draft)
Divya Sethi (Data curation; Formal analysis; Visualization; Writing – review & editing)
Avinash Gaikwad (Data curation; Investigation; Software; Writing – original draft)
Parag Sancheti (Project administration; Resources; Validation; Visualization)
Abhijit Nair (Data curation; Supervision; Visualization; Writing – review & editing)
References
1. Sandhu NS, Capan LM. Ultrasound-guided infraclavicular brachial plexus block. Br J Anaesth. 2002; 89:254–9.

2. Chin KJ, Alakkad H, Adhikary SD, Singh M. Infraclavicular brachial plexus block for regional anaesthesia of the lower arm. Cochrane Database Syst Rev. 2013; 28:CD005487.

3. Klaastad O, Smith HJ, Smedby O, Winther-Larssen EH, Brodal P, Breivik H, et al. A novel infraclavicular brachial plexus block: the lateral and sagittal technique, developed by magnetic resonance imaging studies. Anesth Analg. 2004; 98:252–6.
4. Karmakar MK, Sala-Blanch X, Songthamwat B, Tsui BC. Benefits of the costoclavicular space for ultrasound-guided infraclavicular brachial plexus block: description of a costoclavicular approach. Reg Anesth Pain Med. 2015; 40:287–8.
5. Charbonneau J, Fréchette Y, Sansoucy Y, Echave P. The ultrasound-guided retroclavicular block: a prospective feasibility study. Reg Anesth Pain Med. 2015; 40:605–9.
6. Tran DQ, Charghi R, Finlayson RJ. The “double bubble” sign for successful infraclavicular brachial plexus blockade. Anesth Analg. 2006; 103:1048–9.

7. Li JW, Songthamwat B, Samy W, Sala-Blanch X, Karmakar MK. Ultrasound-guided costoclavicular brachial plexus block: sonoanatomy, technique, and block dynamics. Reg Anesth Pain Med. 2017; 42:233–40.
8. Songthamwat B, Karmakar MK, Li JW, Samy W, Mok LY. Ultrasound-guided infraclavicular brachial plexus block: prospective randomized comparison of the lateral sagittal and costoclavicular approach. Reg Anesth Pain Med. 2018; 43:825–31.
9. Raphael DT, McIntee D, Tsuruda JS, Colletti P, Tatevossian R. Frontal slab composite magnetic resonance neurography of the brachial plexus: implications for infraclavicular block approaches. Anesthesiology. 2005; 103:1218–24.
10. Chhabra A, Thawait GK, Soldatos T, Thakkar RS, Del Grande F, Chalian M, et al. High-resolution 3T MR neurography of the brachial plexus and its branches, with emphasis on 3D imaging. AJNR Am J Neuroradiol. 2013; 34:486–97.

11. Martinoli C, Gandolfo N, Perez MM, Klauser A, Palmieri F, Padua L, et al. Brachial plexus and nerves about the shoulder. Semin Musculoskelet Radiol. 2010; 14:523–46.

12. Grossi P. Brachial plexus block. The anaesthetic line is a guide for new approaches. Minerva Anestesiol. 2001; 67:45–9.
13. Koscielniak-Nielsen ZJ, Rasmussen H, Hesselbjerg L. Pneumothorax after an ultrasound-guided lateral sagittal infraclavicular block. Acta Anaesthesiol Scand. 2008; 52:1176–7.

Fig. 1.
Probe placement and needle insertion along the brachial line. (A) The linear probe is placed parallel to the brachial line that joins the AA, mid-point of the clavicle, and C6 tubercle. (B) The needle is inserted in-plane and in a caudal to cephalad direction along the brachial line. The probe is then tilted medially or laterally to visualize the medial/posterior cords or lateral cord, respectively. The marker of the probe is caudal towards the axilla. AA: axillary artery, OLA: oblique longitudinal axis, SCM-MH: medial head of the sternocleidomastoid muscle, SCM-LH: lateral head of the sternocleidomastoid muscle, C6: Chassaignac's tubercle.
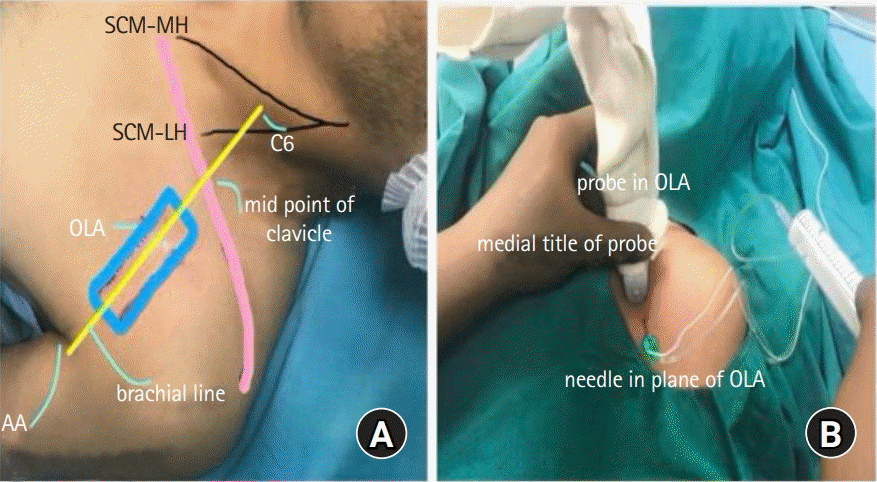
Fig. 2.
Neurostimulation of the brachial cords. (A) The needle tip is placed medial to the AA to evoke an MC or a PC response. (B) The needle tip is placed lateral to the AA to evoke an LC response. The marker is caudal towards the axilla. PMa: pectoralis major, PMi: pectoralis minor, MC: medial cord (yellow), LC: lateral cord (yellow), PC: posterior cord (yellow), AA: axillary artery (red), LA: local anesthetic (blue).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download