Abstract
For over a thousand years, various substances have been applied to the skin to treat pain. Some of these substances have active ingredients that we still use today. However, some have been discontinued due to their harmful effect, while others have been long forgotten. Recent concerns regarding the cardiovascular and renal risk from nonsteroidal anti-inflammatory drugs, and issues with opioids, have resulted in increasing demand and attention to non-systemic topical alternatives. There is increasing evidence of the efficacy and safety of topical agents in pain control. Topical analgesics are great alternatives for pain management and are an essential part of multimodal analgesia. This review aims to describe essential aspects of topical drugs that physicians should consider in their practice as part of multimodal analgesia. This review describes the mechanism of popular topical analgesics and also introduces the most recently released and experimental topical medications.
Go to : 
One of the first concerns historically facing humanity would have been physical pain. It is no exaggeration to say that the history of medicine derived from humans trying to reduce pain. In modern times, these efforts to lessen pain are summed up by the term "multimodal analgesia," which refers to a medical practice using diverse systemic medications with various mechanisms of actions [1,2] along with regional/peripheral block techniques [3].
For thousands of years, a variety of natural materials have been applied to the skin for treating pain. Some of these substances have active components that are still in use today. However, some substances that proved to be harmful have been discontinued, while others have long been forgotten.
Topical analgesics are great alternatives for pain management and an essential part of multimodal analgesia. Healthcare providers and pharmaceutical companies are now reevaluating the effectiveness of potential analgesics and additional safety benefits from one of the oldest routes of drug administration: topical application. A survey reported that 27% of physicians prescribed compounded topical medications for pain relief, and 43% of patients responded favorably to topical agents with minimal side effects [4]. Topical drug administration has many potential benefits, especially in pain presentations that have localized and peripheral components (Table l). The rationale of topical drugs is based on their ability to block or inhibit the pain pathway locally or peripherally, with minimum systemic uptake. Topical analgesic agents are easy to use and have obvious benefits, avoiding systemic adverse effects and drug-drug interactions, which can be observed in systemic drug administration. Hence, these agents are the preferred choice for elderly who take concomitant multidrug therapy for various diseases. In fact, the elderly are the main target population to benefit from topical agents; The American Geriatric Society recommended the use of topical agents for localized neuropathic and non-neuropathic pain [5]. Furthermore, topical agents usually do not require dose titration, as do many systemic agents. For these reasons, the use of topical agents is increasing. Since the United States Food and Drug Administration (FDA) approved topical analgesics, 8% capsaicin and 5% lidocaine patches (LP) to treat postherpetic neuralgia (PHN), many other drugs including ketamine cream, gabapentin gel, topical baclofen, and clonidine gel have been trialed.
Pros and Cons of Topical Drugs
Mechanisms of action for topical treatments include interactions with nociceptive neural networks in the outer layers of the skin through various inflammatory processes. Once applied to the skin, these substances must penetrate the stratum corneum, which can be a significant barrier. Substances that enhance this penetration have evolved in parallel with new topical drugs. Current topical medications with significant past histories that warrant discussion include nonsteroidal anti-inflammatory drugs (NSAIDs), local anesthetic agents, capsaicin, and ketamine. Topical routes of administration may increase due to the advent of new medications targeted to specific pain mechanisms and advances in drug penetration technology through the skin barrier. Furthermore, research on which patients will respond best to these methods of treatment is required.
This review aims to describe the key aspects of topical drugs that physicians should consider for their use as part of multimodal analgesia. The article discusses the differences between topical and transdermal drug delivery, the microstructure of the skin, and the mechanism of action of each medication. The latest topical medications are also described.
Go to : 
The words "transdermal" and "topical" are often used interchangeably. However, it is necessary to distinguish between the two terms. The transdermal delivery of drugs is achieved by the percutaneous absorption of the substance, eventually reaching systemic therapeutic levels comparable with systemic administration. Therefore, transdermal drugs can be administered far from the area of pain and can cause adverse effects similar to systemic medication. Examples of transdermal agents are a sustained-release nicotine patch and long-acting fentanyl patch system. Despite the word 'transdermal,' the effect is primarily 'systemic' [6]. Transdermal delivery of medications serves as a reservoir within or adjacent to the skin, gradually releasing the substance into the systemic circulation leading to significant delays before reaching maximum plasma concentrations, making it a poor choice for sudden exacerbation of chronic pain or acute pain treatment [7]. Conversely, topical drugs target the underlying soft tissue and peripheral nerves at the application site. They exert their therapeutic action at the application site by penetrating the skin via passive diffusion [8]. Topical medications can accumulate at therapeutic concentrations within the local tissue to which they are applied while maintaining a low plasma concentration [8]. Due to the low systemic concentration, topical drugs do not cause adverse systemic reactions or interactions between drugs. Topical medications can potentially have similar efficacy to oral formulations on a locally applied site without the associated systemic side effects.
Go to : 
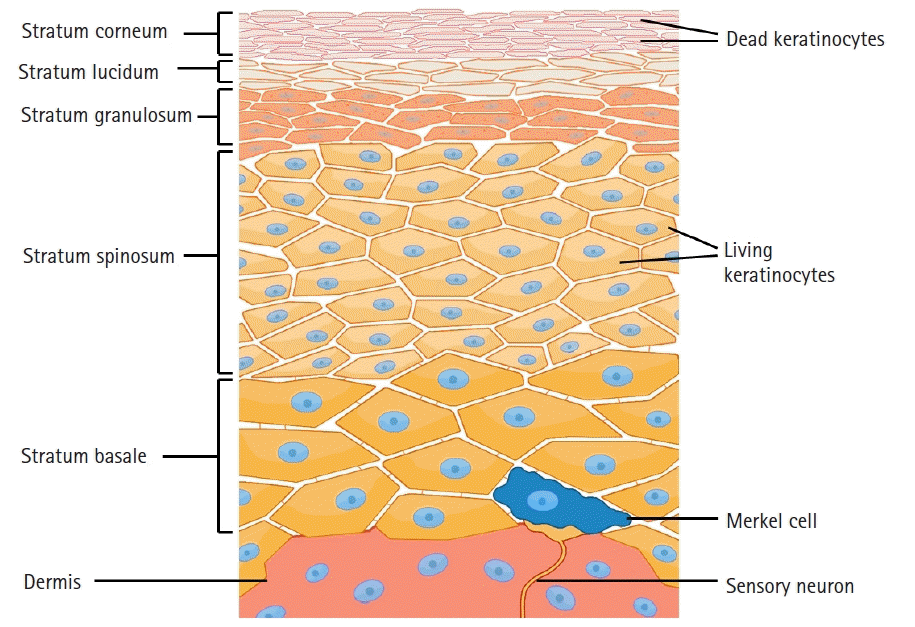
The stratum corneum is the outermost layer of the epidermis, formed with dense, flattened keratinocytes (Fig. 1). This layer functions as a barrier to protect the underlying tissue from dehydration, infection, and chemical/mechanical stress. Topical medication must pass through the stratum corneum of the epidermis to show its effect [8]. After passing through this relatively impermeable barrier, the drugs can access the underlying cutaneous nociceptors: unmyelinated Aδ and C-fibers. The penetration of the stratum corneum is determined by the following essential parameters of the drug: oil/water partition coefficient, dimension, and superficial properties [9]. The stratum corneum is mostly hydrophobic, while the epidermis is predominantly aqueous. Therefore, an ideal topical drug should have a low molecular weight (< 500 Da) [10] and have both hydrophobic and hydrophilic characteristics to pass through the stratum corneum and aqueous epidermis [11].
The differences in the application site (e.g., the variation of the stratum corneum, skin integrity, and the density of appendages) can affect the absorption of topical drugs [12]. The integrity of the skin can be affected by pathological conditions and dehydration of the skin. Furthermore, the increased water content in the stratum corneum of 20–50% can cause swelling of the corneocytes, reducing the compactness of the layer and diffusion resistance [13]. The physical characteristics of the chemical contribute to the absorption of the topical agents. The more lipophilic the drug is, the more it is partitioned into the stratus corneum [14]. Also, the solubility of the molecule in its vehicle can affect the absorption and concentration of the drug in the skin. For a long time, numerous delivery agents have been designed to enhance the bioavailability and the absorbability of topical drugs. For example, lecithin organogels, pluronic gel, and pluronic lecithin organogel have been used [15,16]. Moreover, patch or plaster formulations can be used, which provide additional benefit to traditional topical gels or creams because they can offer continuous and increased absorption [17].
Go to : 
The keratinocytes, which constitute 90% of epidermis cells, are one of the main targets of topical analgesics. While keratinocytes are generally considered as non-excitable cells, they express various signaling molecules. Peripheral injuries induce keratinocytes and blood vessels in the dermis to produce excitatory factors, such as substance P, calcitonin gene-related peptide, and prostaglandin that bind to receptors on nociceptive fibers, resulting in depolarization. There is evidence that keratinocytes have both analgesic and algesic properties involving sensory transduction and modulation of epidermal sensory endings [18]. The analgesic property of keratinocytes express β-endorphins, which are released by the activation of endothelin-1 receptor B and cannabinoid 2 receptors, both expressed in the upper stratum of keratinocytes [19]. The algesic mechanism of keratinocytes involves the production of adenosine triphosphate and the calcitonin gene-related peptide β that are released by the activation of voltage-gated Na+ channels expressed on the keratinocytes [20].
The unmyelinated C and Aδ-fiber, which convey the feeling of pain, are stimulated by noxious stimuli of mechanical, chemical, and thermal inputs. Topical analgesics, such as capsaicin, ketamine, and lidocaine, act mainly on the free nerve endings of the unmyelinated C-fibers. The superficial stratum corneum contains free endings of horizontal distribution, also known as the sub-epidermal nerve net [21]. These endings originate from unmyelinated nerve fibers and are poor in axoplasmic organelles. Their main characteristic is the extensive neural network in the skin, distributed in the stratum corneum next to and inside the epidermis. This arrangement secures its efficacy in collecting the appropriate stimuli.
Go to : 
The oldest topical drugs may have been counterirritants to suppress the perception of pain. Counterirritants (e.g., menthol, camphor, peppermint oil, and garlic) have long been used throughout history. However, it was only recently that their molecular mechanism of action was revealed. Currently, various medications are applied topically (e.g., NSAIDs, local anesthetics, capsaicin, ketamine, and nitroglycerin) to reduce pain.
NSAIDs are the most widely used commercially available topical agent and have diverse formulation. Among the topical agents, NSAIDS have the largest amount of clinical experience and accumulated evidence regarding their effect. Since the total systemic absorption from the topical application is only 3–5% of the oral administration, systemic toxicity from topical NSAIDs is consequently rare [22]. Previous studies have demonstrated that topical NSAIDs could reach sufficient therapeutic concentration; When applied topically, the concentration of ketoprofen was 30-fold higher in the adjacent cartilage than in the plasma. Furthermore, the Cmax values of ketoprofen of the intra-articular tissue were 6.8-fold higher in the topical route compared to oral administration [23]. Topical NSAIDs can be used in various painful conditions, including ophthalmic surgery, mucosal lesions, skin ulcers, strains/sprains, and venous cannulation [24–26]. Current guidelines in the United Kingdom recommend the use of topical NSAIDs ahead of oral administration for hand or knee osteoarthritis in consideration of the risk and benefits of pharmacological treatments [27].
Lidocaine inhibits the voltage-gated Na+ channels and reduces the excitability of cutaneous sensory neurons. In the 1990s, a patch formulation of lidocaine 5% was developed and approved by the United States FDA for the treatment of PHN. Many guidelines recommend LP as first-line therapy for PHN [28,29]. Moreover, it has been increasingly used in other neuropathic conditions [30,31] and many pain-related conditions due to its ease of use and low systemic adverse effects [32,33]. Several studies have supported that LP provide adequate postoperative pain relief after various surgeries, including laparoscopic appendectomy [34], gynecological surgery with midline incision [35], radical prostatectomy [36], and endoscopic discectomy [37]. LP are 10 ⅹ 14 cm sized hydrogel adhesive patches containing 700 mg of lidocaine at a 5% concentration. A maximum of three patches per day is allowed on intact skin, with an on-off interval of 12 hours. On application, lidocaine is continuously released from the patch with 3 ± 2% being absorbed systemically and more than 95% of the lidocaine remaining within the applied patch. Pharmacokinetic studies have demonstrated that the application of 5% LP resulted in systemic absorption of only 63 mg for a single 12-hour period application [38], with a peak plasma concentration of 0.13 μg/ml, which is only approximately 10% of the anti-arrhythmic dose [39]. This limited systemic absorption implies both minimal systemic side effects and minimal systemic analgesic effects, as intravenous lidocaine administration does possess an analgesic effect. Recent advances in patch technology have led to the development of 1.8% LP (ZTlido®, Scilex pharmaceuticals, Inc., USA) and a heat-activated topical lidocaine/tetracaine mixture patch (Synera®, Galen Ltd., UK). The former delivers the same amount of lidocaine as a conventional 5% LP and produces a similar analgesic effect, alongside the advantage of better skin attachment [40]. The latter enhances the delivery of local anesthetics through the skin triggered by local warming and can provide analgesia during superficial skin procedures such as venipuncture [41]. Recently a novel formulation of adhesive compounds for topical anesthetics, the film-forming system, has been developed to solve the problem of poor adherence in conventional patches. This system can also provide a metered-dose application. The film-forming system contains a mixture of the medication, film-forming polymer, and solvent system that, once applied, evaporates, and transforms to a thin film on the application site [42]. More effective topical lidocaine delivery technologies are anticipated in the near future.
Substances for counter-irritation, which stimulate and subsequently desensitize nociceptive sensory neurons, have long been used. Although many of the substances in this group, such as camphor, menthol, and garlic, have a long history of general medical use, they have not been used as widely more recently. Capsaicin is considered to have the best evidence in pain treatment among the agents for counter-irritation. The effect of capsaicin on sensory fibers was first recognized in the early 19th century [43]. Topical capsaicin is currently used for alleviating PHN, painful diabetic polyneuropathy, chronic neck pain, HIV-peripheral neuropathy, post-traumatic, and postoperative chronic neuropathy [44]. This substance is an active ingredient in chili peppers belonging to the genus Capsicum. It is an irritant to mammals and causes a burning sensation in any tissue that comes into contact with it [45]. Capsaicin, as a member of the vanilloid family, binds to receptors and activates transient receptor potential vanilloid-1 (TRPV1), which opens transiently and initiates a depolarization mediated by an influx of Na+ and Ca2+. TRPV1 plays an important role in pain transmission, especially pain during inflammation. It is expressed mainly in the primary sensory neurons with unmyelinated C-fibers. Various molecules and stimuli activate TRPV1, such as acidic or basic pH, high temperature above 42℃, transmembrane voltage, lipids, and protein kinases [46,47]. Once activated, TRPV1 leads to the perception of nociceptive stimuli. A previous study reported that TRPV1 undergoes up-regulation in certain disease processes, explaining the exacerbated pain associated with these conditions [48]. Topically applied capsaicin activates the TRPV1 channels and initiates a depolarization mediated by an influx of Na+ and Ca2+, followed by prolonged desensitization of the local pain nerves through TRPV1 expressing pain nerve fibers [49]. Capsaicin induces depolarization of the nociceptive free nerve endings, mainly the unmyelinated C-fibers with the generation of a resultant action potential that is propagated to the spinal cord and brain, ultimately perceived as a burning, warming sensation [50]. Additionally, capsaicin can lead to mitochondrial dysfunction by overloading the Ca2+ sequestration capabilities of mitochondria. Applying capsaicin in a higher concentration than that needed to activate the TRPV1 receptors leads to direct inhibition of the electron transport chain, eventually leading to mitochondrial destruction [51]. The half-life of capsaicin in the skin is estimated to be 24 hours [52]. When capsaicin is topically applied, it is hard to predict the resulting concentration due to much of it being stored in the stratum corneum. The systemic absorption of the capsaicin 8% patch has been previously reported. A one-hour exposure to capsaicin led to a systemic level of 1.75 ng/ml in a subject who had a treatment area of 924 cm2, a value equivalent to dietary ingestion from chili peppers [53]. Ingesting 5 g of chili pepper contains approximately 27 mg of capsaicin, which results in an average Cmax of 2.5/ml [53]. Consequently, capsaicin 8% patch treatment is safe with low systemic absorption at a similar level to ingesting chili peppers in a standard meal. The major limitation of capsaicin use is its pungent effect on the area of application. This limitation may cause early discontinuation of the drug or reduced patient compliance. Distraction techniques appear to be efficient in alleviating the discomfort, and local cooling after capsaicin patch removal was found to be beneficial in relieving the burning sensation [54]. The most recent capsaicin approved by FDA was an 8% capsaicin patch, indicated for PHN. Low dose formulations in forms of patches, creams, and lotions containing 0.025–0.375% capsaicin for treating neuropathic or musculoskeletal pain are also available.
Ketamine, in sub-anesthetic doses, produces a systemic analgesic effect in chronic pain, mainly due to the blockade of N-methyl-D-aspartate (NMDA) receptors in the central nervous system and inhibition of central sensitization processes [55]. NMDA receptors are also located in peripheral sensory afferent nerve endings and can contribute to pain signaling [56]. Ketamine reduces the amplification of the responses to repeated stimuli. Topically applied ketamine may produce its peripheral anti-nociceptive effect by activating neuronal nitric oxide synthase [57]. Topical ketamine is available in a cream or gel formulation. There have been clinical trials on the effect of topical ketamine in postoperative pain [58] and complex regional pain syndrome [59]. The results of these studies were favorable for the use of ketamine without significant adverse effects. Unfortunately, there are no positive results from double-blind clinical trials for the topical analgesic effect in chronic neuropathic conditions. The major problem of topical ketamine is the risk of improper recreational use. As ketamine can be administered by many routes, there are concerns regarding the illegal recreational use of topical ketamine rectally, leading to undesirable effect [60,61]. Physicians should be cautious and aware of recreational use when prescribing topical ketamine.
NG can be converted to nitric oxide (NO), an anti-inflammatory substance that is endogenously released by activated macrophages [62]. The generated NO can modulate the inflammatory process and produce an analgesic effect similar to the action of cholinergic drugs, via a mechanism directed at nociceptors. Cholinergic agents, such as acetylcholine, can induce analgesia by stimulating the release of NO. In a randomized, placebo-controlled trial, the effect of a 5 mg NG patch was evaluated for three days in relieving shoulder pain. After 48 hours, the NG group had significant pain reduction compared to the unchanged control group [62]. Another randomized, placebo-controlled study demonstrated that topical nitroglycerin was effective for reducing pain from chronic extensor tendinosis [63]. Compared to the placebo group, the topical nitroglycerin group showed significant pain reduction and increased strength of the wrist extensor.
A great variety of other drugs are being developed or are currently used topically. Topical formulations containing various components, such as antidepressants, gabapentin, phenytoin, opioids, cannabinoids, and baclofen, are at various developmental stages [64]. An online survey reported that many clinicians use up to 36 different agents for topical use [4]. However, no absolute standardization has been observed for the majority of compounding substances until now. Moreover, since there are only a few clinical trials available that examine topical agents, it is difficult to confirm the effectiveness of these drugs. Further clinical studies using standardized formulations are needed to demonstrate the effectiveness of these drugs for topical use.
Gabapentin is an anticonvulsant that inhibits the α2δ1 subunit of voltage-gated Ca2+ channels resulting in the reduction of neuropathic pain. Although there are only animal experiments, topical gabapentin gel at a 10% concentration showed similar outcomes to systemic gabapentin in reversing streptozotocin-induced vulvodynia and relieving allodynia in a streptozotocin-induced diabetes mellitus rat model [65]. Topical gabapentin alleviated severe pain from PHN and other neuropathic conditions [66]. A randomized controlled trial reported that topical 6% gabapentin was effective for reducing chronic kidney disease-associated pruritus [67]. Currently, a gel formulation containing gabapentin 6% and lidocaine 5% is commercially available in some countries.
Baclofen, a gamma-aminobutyric acid (GABA)B receptor agonist, reduces Ca2+ membrane conductance and increases K+ conductance to reduce pain [68]. In the peripheral nervous system, GABAB receptors are located in the cutaneous layers on keratinocytes and nerve endings. There have been some reports on the effectiveness of topical baclofen. Patients with vulvodynia treated with a topical baclofen 2% cream, reported significant pain reduction (> 50% improvement) compared to baseline [69]. A formula with a combination of ketamine 1.5%, baclofen 0.8%, and amitriptyline 3% showed promising results and improved symptoms of burning, tingling, and cramping pain in chemotherapy-induced neuropathy [70].
Phenytoin cream, a nonselective voltage-gated Na+ channel stabilizer, and GABAA receptor agonist showed promising results in allodynia reduction. Phenytoin 5% cream decreased allodynia for 8 hours in patients with diabetic neuropathy, while the 10% cream completely relieved symptoms for over 12 hours [71]. This agent was effective in reducing the pain at the episiotomy site [72] and reduced burning pain in patients with small fiber neuropathy in sarcoidosis [73].
Although there is much evidence supporting the use of systemic opioids for various painful conditions, topical opioids have been comparatively poorly investigated. The most common pain relief was achieved in patients with a pressure and malignant wound. However, patients with arterial leg ulcers did not benefit from topical opioids [74]. Preliminary results suggested that a combination of topical morphine and topical cannabinoid could have a synergistic effect [75].
Go to : 
Although topical agents are related to a lower risk of systemic adverse effects than oral/intravenous medications, precautions are still required since the prevalence of hepatic and renal impairment is high in the elderly population. Reduction of renal and hepatic function, and the presence of various comorbidities controlled with multidrug therapy, leads to the use of topical drugs due to safety concerns with systemic drugs. Some topical agents have not been tested in patients with impaired renal or hepatic function, subsequently limiting their use for a broader patient population. The lack of evidence regarding topical medications in this population demands large-scaled safety trials. The use of topical agents has certain drawbacks. Topical analgesics should first be used on a small area of skin and thus not be used in conditions such as loss of skin integrity to minimize the risk of toxicity. Another significant limitation is the pungency of counterirritants such as capsaicin. It can lead to lowered patient compliance or other side effects when improperly applied. Additionally, off-label use of topical agents requires special precautions due to the high prevalence of renal and hepatic impairments in the elderly.
Also, the desired concentration of a drug remains a limitation despite the availability of various medications for topical use. It is necessary to improve the methods of drug delivery to penetrate the skin barrier efficiently while providing an effective therapeutic dose to reduce pain.
Go to : 
Topical medications can be used alone or in combination with two or more agents. The combinations of different drug classes can be combined to create topical formulations that can target different mechanisms of action and possibly result in a synergistic analgesic effect. Previous studies have reported that a combination of topical morphine and topical cannabinoid can enhance the anti-allodynic effects while reducing the central effect of the opioids [75]. This result could lead to the development of newer combination agents. Further studies are needed to assess the efficacy and feasibility of topical compound formulations.
Besides, new agents should be trialed for use as topical formulations. For example, although it has not been used as a topical agent due to insufficient transdermal absorption, resiniferatoxin (RTX) could potentially be an efficacious topical drug. RTX is a potent capsaicin analog, which is extracted from the resin of the Euphorbia cactus [76]. RTX slows down depolarization and reduces Ca2+ influx into C-fibers [77]. RTX induces extremely prolonged channel opening and Ca2+ influx, resulting in cytotoxicity in TRPV1-positive pain fibers [78]. The sustained Ca2+ influx induced by RTX selectively destroys the peripheral nerve endings or the entire sensory neurons containing TRPV1 while sparing the motor, proprioceptive, and other somatosensory functions [79]. This selective neuro-ablation effect has been labeled as "molecular neurosurgery" [80]. There are promising results of RTX in the treatment of osteoarthritis [81]. However, permeation of RTX into the skin is inadequate, and research on enhancing its dermal absorbability is needed. If this research is successful, topical RTX could play an important role in topical analgesia.
Go to : 
Recent studies have broadened our understanding of the various mechanism through which topical medications result in pain relief. Due to the complex nature of pain, topical analgesia should be recruited as part of multimodal pain treatment. Enhancing the understanding of topical medications would be important to ensure optimal pain practice for patients requiring diverse, multimodal analgesic treatment options.
In summary, topical agents are a simple but effective method for treating pain and can play a crucial role in multimodal pain management. Further research is needed to elucidate the role and effectiveness of topical analgesics, especially when combined with other treatment modalities.
Go to : 
ACKNOWLEDGMENTS
The authors thank Ms. Mihee Park of Seoul National Bundang Hospital for her illustration work in this study.
Go to : 
Notes
Author Contributions
Eunjoo Choi (Writing – original draft)
Francis Sahngun Nahm (Conceptualization; Project administration; Writing – review & editing)
Woong Ki Han (Writing – review & editing)
Pyung-Bok Lee (Supervision)
Jihun Jo (Visualization)
Go to : 
References
1. Kim KH, Seo HJ, Abdi S, Huh B. All about pain pharmacology: what pain physicians should know. Korean J Pain. 2020; 33:108–20.

2. Govil N, Parag K, Arora P, Khandelwal H, Singh A. Perioperative duloxetine as part of a multimodal analgesia regime reduces postoperative pain in lumbar canal stenosis surgery: a randomized, triple blind, and placebo-controlled trial. Korean J Pain. 2020; 33:40–7.

3. Hong B, Bang S, Chung W, Yoo S, Chung J, Kim S. Multimodal analgesia with multiple intermittent doses of erector spinae plane block through a catheter after total mastectomy: a retrospective observational study. Korean J Pain. 2019; 32:206–14.

4. Ness TJ, Jones L, Smith H. Use of compounded topical analgesics- results of an Internet survey. Reg Anesth Pain Med. 2002; 27:309–12.
5. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009; 57:1331–46.
6. Durand C, Alhammad A, Willett KC. Practical considerations for optimal transdermal drug delivery. Am J Health Syst Pharm. 2012; 69:116–24.

7. Jeal W, Benfield P. Transdermal fentanyl. A review of its pharmacological properties and therapeutic efficacy in pain control. Drugs. 1997; 53:109–38.
8. Stanos SP. Topical agents for the management of musculoskeletal pain. J Pain Symptom Manage. 2007; 33:342–55.

10. Brown MB, Martin GP, Jones SA, Akomeah FK. Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv. 2006; 13:175–87.

11. Vaile JH, Davis P. Topical NSAIDs for musculoskeletal conditions. A review of the literature. Drugs. 1998; 56:783–99.
12. Baroli B. Penetration of nanoparticles and nanomaterials in the skin: fiction or reality? J Pharm Sci. 2010; 99:21–50.

13. Van Hal DA, Jeremiasse E, Junginger HE, Spies F, Bouwstra JA. Structure of fully hydrated human stratum corneum: a freeze-fracture electron microscopy study. J Invest Dermatol. 1996; 106:89–95.

14. Moss GP, Dearden JC, Patel H, Cronin MT. Quantitative structure-permeability relationships (QSPRs) for percutaneous absorption. Toxicol In Vitro. 2002; 16:299–317.

15. Kumar R, Katare OP. Lecithin organogels as a potential phospholipid-structured system for topical drug delivery: a review. AAPS PharmSciTech. 2005; 6:E298–310.

16. Franckum J, Ramsay D, Das NG, Das SK. Pluronic lecithin organogel for local delivery of anti-inflammatory drugs. Int J Pharm Compd. 2004; 8:101–5.
17. Assandri A, Canali S, Giachetti C. Local tolerability and pharmacokinetic profile of a new transdermal delivery system, diclofenac hydroxyethylpyrrolidine plaster. Drugs Exp Clin Res. 1993; 19:89–95.
18. Keppel Hesselink JM, Kopsky DJ, Bhaskar AK. Skin matters! The role of keratinocytes in nociception: a rational argument for the development of topical analgesics. J Pain Res. 2017; 10:1–8.

19. Peppin JF, Albrecht PJ, Argoff C, Gustorff B, Pappagallo M, Rice FL, et al. Skin matters: a review of topical treatments for chronic pain. Part one: skin physiology and delivery systems. Pain Ther. 2015; 4:17–32.

20. Zhao P, Barr TP, Hou Q, Dib-Hajj SD, Black JA, Albrecht PJ, et al. Voltage-gated sodium channel expression in rat and human epidermal keratinocytes: evidence for a role in pain. Pain. 2008; 139:90–105.

22. Heyneman CA, Lawless-Liday C, Wall GC. Oral versus topical NSAIDs in rheumatic diseases: a comparison. Drugs. 2000; 60:555–74.
23. Rolf C, Engström B, Beauchard C, Jacobs LD, Le Liboux A. Intra-articular absorption and distribution of ketoprofen after topical plaster application and oral intake in 100 patients undergoing knee arthroscopy. Rheumatology (Oxford). 1999; 38:564–7.

24. Kumar S, Sanjeev O, Agarwal A, Shamshery C, Gupta R. Double blind randomized control trial to evaluate the efficacy of ketoprofen patch to attenuate pain during venous cannulation. Korean J Pain. 2018; 31:39–42.

25. Rodriguez-Merchan EC. Topical therapies for knee osteoarthritis. Postgrad Med. 2018; 130:607–12.

26. Derry S, Wiffen PJ, Kalso EA, Bell RF, Aldington D, Phillips T, et al. Topical analgesics for acute and chronic pain in adults- an overview of cochrane reviews. Cochrane Database Syst Rev. 2017; 5:CD008609.
27. Conaghan PG, Dickson J, Grant RL. Care and management of osteoarthritis in adults: summary of NICE guidance. BMJ. 2008; 336:502–3.

28. Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010; 17:1113–23.

29. Dworkin RH, O'Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010; 85:S3–14.

30. Meier T, Wasner G, Faust M, Kuntzer T, Ochsner F, Hueppe M, et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain. 2003; 106:151–8.

31. Barbano RL, Herrmann DN, Hart-Gouleau S, Pennella-Vaughan J, Lodewick PA, Dworkin RH. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Arch Neurol. 2004; 61:914–8.

32. Cheng YJ. Lidocaine skin patch (lidopat® 5%) is effective in the treatment of traumatic rib fractures: a prospective double-blinded and vehicle-controlled study. Med Princ Pract. 2016; 25:36–9.

33. Gammaitoni AR, Galer BS, Onawola R, Jensen MP, Argoff CE. Lidocaine patch 5% and its positive impact on pain qualities in osteoarthritis: results of a pilot 2-week, open-label study using the neuropathic pain scale. Curr Med Res Opin. 2004; 20 Suppl 2:S13–9.

34. Lee W, Hahn K, Hur J, Kim Y. Effect of topical lidocaine patch on postoperative pain management in laparoscopic appendectomy: a randomized, double-blind, prospective study. J Laparoendosc Adv Surg Tech A. 2018; 28:1061–7.

35. Lau LL, Li CY, Lee A, Chan SK. The use of 5% lidocaine medicated plaster for acute postoperative pain after gynecological surgery: a pilot randomized controlled feasibility trial. Medicine. 2018; 97:e12582.
36. Habib AS, Polascik TJ, Weizer AZ, White WD, Moul JW, ElGasim MA, et al. Lidocaine patch for postoperative analgesia after radical retropubic prostatectomy. Anesth Analg. 2009; 108:1950–3.

37. Kim KH. Use of lidocaine patch for percutaneous endoscopic lumbar discectomy. Korean J Pain. 2011; 24:74–80.

38. Campbell BJ, Rowbotham M, Davies PS, Jacob P, Benowitz NL. Systemic absorption of topical lidocaine in normal volunteers, patients with postherpetic neuralgia, and patients with acute herpes zoster. J Pharm Sci. 2002; 91:1343–50.

39. Argoff CE. Targeted topical peripheral analgesics in the management of pain. Curr Pain Headache Rep. 2003; 7:34–8.

40. ZTlido--a new lidocaine patch for postherpetic neuralgia. Med Lett Drugs Ther. 2019; 61:41–3.
41. Pastore MN, Kalia YN, Horstmann M, Roberts MS. Transdermal patches: history, development and pharmacology. Br J Pharmacol. 2015; 172:2179–209.

42. Tran TTD, Tran PHL. Controlled release film forming systems in drug delivery: the potential for ffficient drug delivery. Pharmaceutics. 2019; 11:290.
43. Bley KR. Recent developments in transient receptor potential vanilloid receptor 1 agonist-based therapies. Expert Opin Investig Drugs. 2004; 13:1445–56.

44. Derry S, Rice AS, Cole P, Tan T, Moore RA. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013; 2:CD007393.

46. Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998; 21:531–43.

47. Dhaka A, Uzzell V, Dubin AE, Mathur J, Petrus M, Bandell M, et al. TRPV1 is activated by both acidic and basic pH. J Neurosci. 2009; 29:153–8.

48. Niiyama Y, Kawamata T, Yamamoto J, Omote K, Namiki A. Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience. 2007; 148:560–72.

49. Watanabe N, Horie S, Spina D, Michael GJ, Page CP, Priestley JV. Immunohistochemical localization of transient receptor potential vanilloid subtype 1 in the trachea of ovalbumin-sensitized guinea pigs. Int Arch Allergy Immunol. 2008; 146 Suppl 1:28–32.

50. Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011; 107:490–502.

51. Bley K. Effects of topical capsaicin on cutaneous innervation: implications for pain management. Open Pain J. 2013; 6:81–94.

52. Pershing LK, Reilly CA, Corlett JL, Crouch DJ. Effects of vehicle on the uptake and elimination kinetics of capsaicinoids in human skin in vivo. Toxicol Appl Pharmacol. 2004; 200:73–81.

53. Chaiyasit K, Khovidhunkit W, Wittayalertpanya S. Pharmacokinetic and the effect of capsaicin in capsicum frutescens on decreasing plasma glucose level. J Med Assoc Thai. 2009; 92:108–13.
54. England J, Wagner T, Kern KU, Roth-Daniek A, Sell A. The capsaicin 8% patch for peripheral neuropathic pain. Br J Nurs. 2011; 20:926–31.

55. Knezevic NN, Tverdohleb T, Nikibin F, Knezevic I, Candido KD. Management of chronic neuropathic pain with single and compounded topical analgesics. Pain Manag. 2017; 7:537–58.

57. Gordh T, Karlsten R, Kristensen J. Intervention with spinal NMDA, adenosine, and NO systems for pain modulation. Ann Med. 1995; 27:229–34.

58. Tekelioglu UY, Apuhan T, Akkaya A, Demirhan A, Yildiz I, Simsek T, et al. Comparison of topical tramadol and ketamine in pain treatment after tonsillectomy. Paediatr Anaesth. 2013; 23:496–501.

59. Finch PM, Knudsen L, Drummond PD. Reduction of allodynia in patients with complex regional pain syndrome: a double-blind placebo-controlled trial of topical ketamine. Pain. 2009; 146:18–25.

60. Cohen CE, Giles A, Nelson M. Sexual trauma associated with fisting and recreational drugs. Sex Transm Infect. 2004; 80:469–70.

61. Kalsi SS, Wood DM, Dargan PI. The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use. Emerg Health Threats J. 2011; 4:7107.

62. Berrazueta JR, Losada A, Poveda J, Ochoteco A, Riestra A, Salas E, et al. Successful treatment of shoulder pain syndrome due to supraspinatus tendinitis with transdermal nitroglycerin. A double blind study. Pain. 1996; 66:63–7.

63. Paoloni JA, Appleyard RC, Nelson J, Murrell GA. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003; 31:915–20.
65. Shahid M, Subhan F, Ahmad N, Ali G, Akbar S, Fawad K, et al. Topical gabapentin gel alleviates allodynia and hyperalgesia in the chronic sciatic nerve constriction injury neuropathic pain model. Eur J Pain. 2017; 21:668–80.

66. Hiom S, Patel GK, Newcombe RG, Khot S, Martin C. Severe postherpetic neuralgia and other neuropathic pain syndromes alleviated by topical gabapentin. Br J Dermatol. 2015; 173:300–2.

67. Aquino TMO, Luchangco KAC, Sanchez EV, Verallo-Rowell VM. A randomized controlled study of 6% gabapentin topical formulation for chronic kidney disease-associated pruritus. Int J Dermatol. 2020; 59:955–61.

68. Ong J, Kerr DI. Recent advances in GABAB receptors: from pharmacology to molecular biology. Acta Pharmacol Sin. 2000; 21:111–23.
69. Kopsky DJ, Keppel Hesselink JM. Neuropathic pain as a result of acromegaly, treated with topical baclofen cream. J Pain Symptom Manage. 2013; 46:e4–5.
70. Barton DL, Wos EJ, Qin R, Mattar BI, Green NB, Lanier KS, et al. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support Care Cancer. 2011; 19:833–41.

71. Kopsky DJ, Keppel Hesselink JM. Topical phenytoin for the treatment of neuropathic pain. J Pain Res. 2017; 10:469–73.

72. Rashidi F, Sehhati F, Ghojazadeh M, Javadzadeh Y, Haghsaie M. The effect of phenytoin cream in comparison with betadine solution on episiotomy pain of primiparous women. J Caring Sci. 2012; 1:61–5.
73. Hesselink JM, Kopsky DJ. Topical phenytoin cream reduces burning pain due to small fiber neuropathy in sarcoidosis. J Anesth Pain Med. 2017; 2:1–3.

74. Graham T, Grocott P, Probst S, Wanklyn S, Dawson J, Gethin G. How are topical opioids used to manage painful cutaneous lesions in palliative care? A critical review. Pain. 2013; 154:1920–8.

75. Yesilyurt O, Dogrul A, Gul H, Seyrek M, Kusmez O, Ozkan Y, et al. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003; 105:303–8.

76. Szallasi A. Vanilloid (capsaicin) receptors in health and disease. Am J Clin Pathol. 2002; 118:110–21.

78. Kárai LJ, Russell JT, Iadarola MJ, Oláh Z. Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J Biol Chem. 2004; 279:16377–87.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download