Abstract
Pain management plays a fundamental role in enhanced recovery after surgery pathways. The concept of multimodal analgesia in providing a balanced and effective approach to perioperative pain management is widely accepted and practiced, with regional anesthesia playing a pivotal role. Nerve block techniques can be utilized to achieve the goals of enhanced recovery, whether it be the resolution of ileus or time to mobilization. However, the recent expansion in the number and types of nerve block approaches can be daunting for general anesthesiologists. Which is the most appropriate regional technique to choose, and what skills and infrastructure are required for its implementation? A multidisciplinary team-based approach for defining the goals is essential, based on each patient's needs, and incorporating patient, surgical, and social factors. This review provides a framework for a personalized approach to postoperative pain management with an emphasis on regional anesthesia techniques.
The importance of appropriately managed postoperative pain is well-established. However, further improvements can still be made [1,2]. Despite advances in analgesics and multimodal pain regimens, patients still report significant postoperative pain and anxieties related to their pain control in the perioperative period [3]. Poorly controlled pain can have significant sequelae, predisposing patients to pulmonary and cardiac complications, and increasing the risk of poor wound healing. Increased wound sensitivity leads to respiratory muscle splinting, immobilization, and atelectasis. Sympathetic stimulation leads to tachycardia, hypertension, and increased oxygen consumption, which may provoke coronary ischemia in susceptible individuals. Furthermore, prolonged postoperative pain leads to fear, helplessness, and demoralization, reducing patients' engagement with their recovery and reducing satisfaction [4,5].
Such psychological implications are a result of peripheral tissue injury as well as alterations in the central nervous system. Unabated nociceptive signals may lead to changes in the dorsal horn and central processing of afferent stimuli, intensifying the propagation of their transmission. These changes contribute to the development of persistent postsurgical pain (PPSP), which is now recognized as a common and significant health burden [6,7]. Inadequately controlled pain tends to increase the length of post-anesthesia care unit/hospital stay and also increases the risk of hospital readmission, resulting in significant economic impact. In contrast, patients who have well-controlled pain in the postoperative period are less likely to seek additional healthcare interventions after discharge and are more likely to have superior functional outcomes and a faster return to normal activities of daily living [5].
In addition, pain management plays a fundamental role in enhanced recovery after surgery (ERAS) pathways [8]. The concept of multimodal analgesia in providing a balanced and effective approach to perioperative pain management is widely accepted and practiced, with regional anesthesia playing a pivotal role [2,8]. Nerve block techniques can be utilized to achieve the ERAS goals, whether it be the resolution of ileus or time to mobilization. However, the recent increase in the number and types of nerve block approaches can be daunting to general anesthesiologists. Which is the most appropriate regional technique to choose, and what skills and infrastructure are required for its implementation? A multidisciplinary team-based approach for defining the goals is essential, based on each patient's needs, and incorporating patient, surgical, and social factors. This review provides a framework for a personalized approach to postoperative pain management with an emphasis on regional anesthesia techniques.
The recent guidelines on postoperative pain management created jointly by multiple societies advocate for the use of site-specific regional anesthetic techniques (strong recommendation, high-quality evidence) as part of a multimodal analgesic regimen [2], which is effective in several surgical procedures including thoracotomy, joint replacement surgery, and cesarean sections. Similarly, the panel also recommended continuous perineural local anesthetic infusion techniques for those patients who are likely to have prolonged pain in the postoperative period (strong recommendation, moderate quality of evidence) [2].
There has been a recent shift in regional anesthesia away from continuous neuraxial techniques, at least in part due to ERAS protocols. Although epidural analgesia still has a role in major thoracic and abdominal procedures, there has been a trend toward the use of peripheral regional anesthetic techniques instead. This has occurred along with a concurrent increase in less invasive surgical procedures (also endorsed by ERAS protocols) and offers the advantages of more hemodynamic stability and less motor impairment [2,9]. The increased use of oral anticoagulants and the need for postoperative anticoagulation has also limited the use of neuraxial techniques. Moreover, recent meta-analyses show that the previous benefits of postoperative epidural analgesia may be less promising today when compared to less invasive alternatives [10].
Unilateral selective nerve blocks can surpass traditional neuraxial techniques in certain patient populations and may be more appropriate in the ambulatory/ERAS setting where the onus is on expediting recovery and facilitating discharge [11,12]. Several options can specifically target the operative area while minimizing unwanted sensory deficit and motor weakness. For major extremity surgery such as total knee arthroplasty (TKA), adductor canal blocks combined with posterior compartment blocks have helped patients achieve adequate analgesia while meeting the goals of physiotherapy [13]. Transversus abdominis plane (TAP) blocks, rectus sheath blocks, and other emerging blocks such as the erector spinae plane (ESP) block also show promise for truncal procedures. These newer techniques may provide acceptable levels of analgesia with fewer side effects and higher patient satisfaction compared to established standards [14].
The multimodal approach to pain management is integral to ERAS pathways [15], which are designed to improve perioperative patient care and recovery after surgery and reduce hospital length of stay. Early mobilization is an important ERAS goal, and the use of site-specific regional techniques rather than epidurals may help to achieve this. In addition, ERAS pathways place significant emphasis on measures to reduce opioid use to hasten ileus resolution and reduce opioid-related side effects [16]. In light of the opioid epidemic in North America, there is an even greater need for techniques to reduce perioperative opioid use. Opioid over-prescribing in the perioperative period can lead to prolonged postoperative opioid use and misuse, leading to tolerance, dependence, and opioid-induced hyperalgesia [17,18]. As well as nonopioid analgesics such as ketamine, intravenous lidocaine, and gabapentinoids, regional anesthesia has been shown to reduce intra- and postoperative opioid use [18,19].
The shift toward peripheral regional anesthetic techniques has largely been driven by the advent of ultrasound-guided regional anesthesia (UGRA). Ultrasound has made regional anesthesia safer, more efficient, and more accessible to general anesthesiologists [20]. UGRA provides real-time visualization and targeting of major nerves that were previously located with landmark-based "blind" techniques (e.g., nerve stimulation, loss of resistance, paresthesia). Currently, there is a movement toward even higher precision novel blocks in a quest for locating individual nerves and fascial planes as ultrasound technology continues to improve. However, the evidence for the widespread adoption of these novel techniques is still to be determined [21]. In addition, these highly specialized novel techniques risk excluding general anesthesiologists who have not subspecialized in regional anesthesia. Greater institutional acceptance and adoption may be achieved with the use of a few evidence-based techniques [22].
We believe the incorporation of regional anesthesia into a patient's perioperative journey must be consistently applied, incorporating factors specific to the patient, the intended surgery, and the resources available in the institution, thereby providing individualized patient-centered care.
Each individual responds differently to noxious stimuli, and so it is no surprise that the same surgery will evoke varied pain responses in different patients, despite the seemingly similar pain generator.
When considering the most appropriate regional anesthetic technique for postoperative pain management, anesthesiologists must estimate the degree of postoperative pain the patient will experience in response to the surgical stimulus. It is well recognized that certain demographic and psychosocial characteristics predispose patients to higher levels of postoperative pain. Younger females and those with a tendency toward catastrophizing and neuroticism are more likely to experience greater pain after the same surgery [6,23–26]. Severe and poorly controlled postoperative pain, as well as prolonged duration, are both associated with the development of PPSP [23]. Other important psychological factors associated with both severe acute pain and PPSP that should be considered are anxiety, depression, and chronic stress [24,25,27].
One of the most important predictors of postoperative pain is pre-existing pain [23–26,28]. Even with minor procedures in the ambulatory setting, patients with pre-existing pain syndromes may experience postoperative pain severe enough to warrant hospital admission [29]. A subset of this population which pose a particular challenge are those patients on preoperative analgesics with baseline opioid tolerance [24]. The preoperative identification of patients with these characteristics can allow for the appropriate planning and implementation of multimodal analgesia techniques, including nerve blocks. A regional anesthetic technique, particularly in these patients, can act in conjunction with other components of the multimodal regimen to reduce acute postoperative pain scores, and the transition from acute to PPSP, aligning with postoperative goals. Such planned aggressive management of these patients' pain reduces central sensitization, which can occur during periods of high-intensity pain [23]. Having an open discussion with the patient and surgical team in these cases is essential. It is not uncommon in caring for patients with chronic pain to prioritize analgesia over other goals such as early ambulation and discharge.
Two emerging areas in our understanding of variable pain responsiveness are genetics and epigenetics. We know that those patients who have a heightened response to certain stimuli preoperatively are more likely to experience higher levels of postoperative pain [6,23], which would suggest that those with pre-surgical sensitization (and evidence of hyperalgesia and allodynia) should be identified early. There is currently no convincing evidence that gene mutations are associated with an increased pain response. However, some data suggest that there may be a link to single mutations in certain genes (e.g., catechol-O-methyltransferase, opioid receptor mu 1, and guanosine-5'-triphosphate cyclohydrolase 1) [7]. There is a role for epigenetics and its contribution to the development of PPSP, which infers that a patient's environment can contribute to the expression (or non-expression) of certain genes involved in pain modulation [23]. Future developments in this field will allow anesthesiologists to potentially identify at-risk individuals in advance and plan for targeted analgesic therapy.
A patient's comorbidities also play a role in postoperative pain management needs. The use of regional analgesia generally reduces the requirement for systemic medications, including opioids. This can be beneficial in those with renal impairment who can experience prolonged effects of opioids due to altered opioid pharmacokinetics [30]. Similarly, reduced opioid consumption can result in less respiratory depression and reduced functional capacity. This is especially important for those with respiratory comorbidities, who are at a higher risk of respiratory sequelae in the postoperative period, and for whom targeted effective pain management with regional techniques is beneficial [30,31]. Neuraxial analgesia for major thoracic and abdominal procedures tends to blunt cardio-acceleratory response and sympathetic activation. This can reduce the risk of myocardial ischemia in patients with coronary artery disease by improving the myocardial oxygen supply-demand ratio, as long as hypotension is avoided [30].
However, it is important to recognize the altered metabolism of local anesthetic drugs in patients with end-stage liver and renal disease, who may need their dosing regimen altered. Similarly, some comorbidities may influence the anesthesiologist's decision regarding specific regional anesthetic techniques. For example, choosing an alternative technique instead of an interscalene nerve block for shoulder surgery can result in better preservation of vital capacity, which may be a relevant consideration in certain patients with significant pre-existing respiratory impairment [32]. Another patient-specific consideration is the requirement for the early resumption of anticoagulation postoperatively with novel oral anticoagulants, which precludes the use of an epidural or deep plexus catheter technique [33].
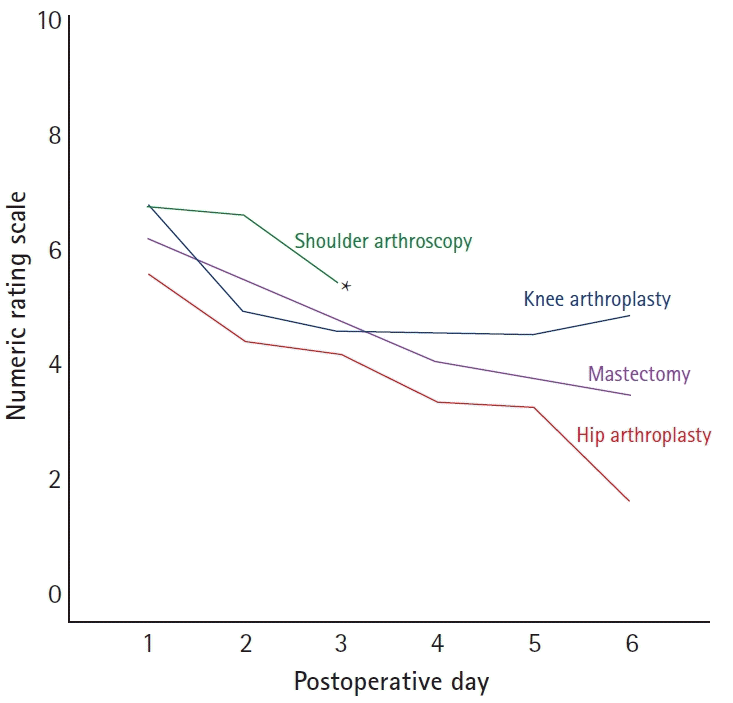
Pain management should be procedure-specific. Knowing the character and duration of pain a patient will encounter in the postoperative period has an important bearing on the ideal regional analgesic technique for them. Different surgeries have different pain trajectories (Fig. 1) [34], and so anesthesiologists must provide each patient with the right intervention at the right time for the right duration.
An open procedure with a large incision can be expected to induce more pain than a minimally invasive procedure. However, patients can experience severe pain even after ambulatory surgery, particularly with orthopedic, urologic, and general surgeries [35]. Certain types of surgery are particularly associated with increased acute postoperative pain and even increased PPSP. These include herniorrhaphy, mastectomy, TKA, limb amputation, thoracotomy, and cesarean sections [7,25]. Patients undergoing these procedures need early identification and aggressive multimodal pain management, including regional nerve blocks. Particular techniques, such as a paravertebral block, may help to prevent PPSP after breast surgery [36]. The surgical technique in itself is also important. Avoiding nerve injury with careful dissection and different surgical approaches, such as avoidance of injury to the intercostobrachial nerve during mastectomy and avoiding the posterolateral approach with a thoracotomy, may decrease chronic pain [6,25]. Therefore, anesthesiologists must understand the technique and approach of the surgical team with whom they are working on a day-to-day basis and plan for procedures collaboratively in advance.
The regional anesthetic technique and local anesthetic chosen should match the predicted pain trajectory of the surgery. Certain procedures result in postoperative pain that far outlast the effects of a single injection peripheral nerve block (sPNB). Adjuvants added to long-acting local anesthetics in sPNB may prolong analgesia and may be beneficial in procedures with an intermediate duration of pain [14,20,37]. However, the only reliable technique that provides analgesia for several days is a continuous peripheral nerve block (cPNB). Perineural catheters can reduce pain scores and opioid consumption in comparison to sPNB [38]. For TKA in particular, a review of postoperative pain suggests that pain scores do not fall below four on a numeric rating scale (0: no pain, 10: worst possible pain) until after postoperative day seven [34]. It is clear that for these patients, an sPNB technique may be inadequate. Similarly, postoperative pain scores after mastectomy, hip arthroplasty, and shoulder arthroscopy all suggest that patients undergoing these procedures have high pain scores for at least three postoperative days, and would, therefore, benefit from a cPNB technique [34]. Unfortunately, the hope of liposomal formulations of local anesthetics in sPNB as a substitute for cPNB techniques has not been realized [20,34,39].
With each surgical procedure comes a different set of postoperative recovery goals on a different timeline. The regional anesthesiologist needs to be cognizant of and work in line with these goals. For example, in lower limb joint arthroplasty, the analgesic technique should allow for the patient to participate in physiotherapy within a day to maximize the functional outcomes. In same-day discharge arthroplasty cases, the technique must also minimize muscle weakness and fall risk, particularly at home. For TKA, more distal nerve block techniques, either at or proximal to the anatomical adductor canal, can offer effective analgesia with quadriceps sparing to allow for early physical therapy [13].
Targeted regional analgesia can also reduce unwanted adverse effects after some surgeries. A paravertebral block may offer equivalent analgesic benefit to a thoracic epidural, with lower rates of urinary retention and hypotension in those undergoing thoracic surgery [40–42]. Fascial plane blocks, such as TAP, rectus sheath, or ESP blocks, can provide analgesia for abdominal procedures while avoiding sympathetic block and hypotension encountered with a thoracic epidural and the risk of epidural hematoma in patients with coagulopathy [20].
As we manage patients as part of a multidisciplinary team, the decision regarding which (if any) regional analgesia technique is best must be agreed upon by the surgeons, anesthesiologists, and of course, the patients themselves. There may be resistance among some surgeons regarding the use of regional analgesia techniques in cases when there is the potential for surgical nerve damage or compartment syndrome. While these may be valid concerns, especially with traumatic injuries of the forearm and lower limbs, robust evidence is lacking. No convincing studies have shown that regional analgesia delays the diagnosis of compartment syndrome [43]. Nevertheless, institutional experience can determine a surgical department's willingness to incorporate regional analgesia in these controversial situations and will affect the number of tools for acute pain management available for the anesthesiologist and acute pain medicine specialist.
The successful implementation of regional anesthesia for postoperative pain management requires proper resources and infrastructure [44]. The infrastructure to support these techniques must be established and embraced by the multidisciplinary team, including anesthesiologists, surgeons, nurses, physiotherapists, and occupational therapists. Evidence-based techniques guided by the procedures performed at the institution should be selected to provide safe, consistent care. Anesthesiologists must be proficient in performing these techniques and lead the development of protocols for patient management in the postoperative period. Ongoing education of all team members is essential along with a process for evaluating the quality of care and patient safety.
Techniques such as epidurals and cPNB require a dedicated acute pain service not only to lead these initiatives but to manage patients with these modalities. Staff on the ward need to be confident and capable of managing these techniques and be able to recognize any adverse effects [38,45]. With the growing pressure to shorten hospital stays, many patients may be discharged with cPNB techniques or the effects of a sPNB in place. Therefore, appropriate patient selection and education are critical [34].
Additional resources are required for regional analgesia include staff, capital, equipment, and consumable costs. Institutions require anesthesiologists trained in performing these techniques [22]. In addition, specialized nurses are invaluable in helping to maintain these programs and provide ongoing staff education. Capital costs include ultrasounds, infusion pumps, and potentially a dedicated block room to perform these procedures efficiently. Specialized epidural and block kits with needles, ultrasound machines, catheters, and local anesthetic solutions are also additional expenses. However, these costs may be offset by improved patient outcomes, reduced complications, shorter lengths of stays, and less downstream healthcare utilization post-discharge [46].
Unfortunately, poor access and inequitable distribution of healthcare resources may limit postoperative analgesic options for patients. There needs to be a minimum standard of care established that must be met at all institutions regardless of each patient's socioeconomic status. This should be a priority not only with postoperative analgesia but across the spectrum of healthcare delivery [47]. Local anesthetic, in some form, should be part of every multimodal pain management strategy. In situations where sophisticated regional analgesia is not available, this may mean meticulous layer by layer local anesthetic infiltration by the surgeon during wound closure or adjustments in the regional anesthesia technique if other limitations exist such as using adjuvants to prolong sPNB [14,20] or elastomeric infusion pumps if dedicated programmable pumps are not available [48].
Increasing patient access to a range of regional anesthesia options for various surgeries starts with having a critical mass of anesthesiologists willing and able to perform the procedures safely and effectively. Then, these techniques need to be incorporated into standardized surgical pathways. Even though it is well-established that paravertebral blockade is effective for pain management after breast surgery, some general anesthesiologists may be hesitant to attempt this due to a lack of experience and fear of complications. Education and training play a major role in increasing patient access to robust multimodal analgesia involving regional nerve blocks. In this example, the ESP block may be an attractive alternative to paravertebral block because the deposition of local anesthetic superficial to the paravertebral space may be perceived as easier and safer by the general anesthesiologist [47]. Given the opioid epidemic, educators have recognized the importance of training every anesthesiologist in a basic armamentarium of regional analgesia options. With a consistent curriculum, every anesthesiologist can achieve the competence and confidence to perform a basic set of nerve blocks and learn to incorporate regional analgesic techniques into routine perioperative care [22].
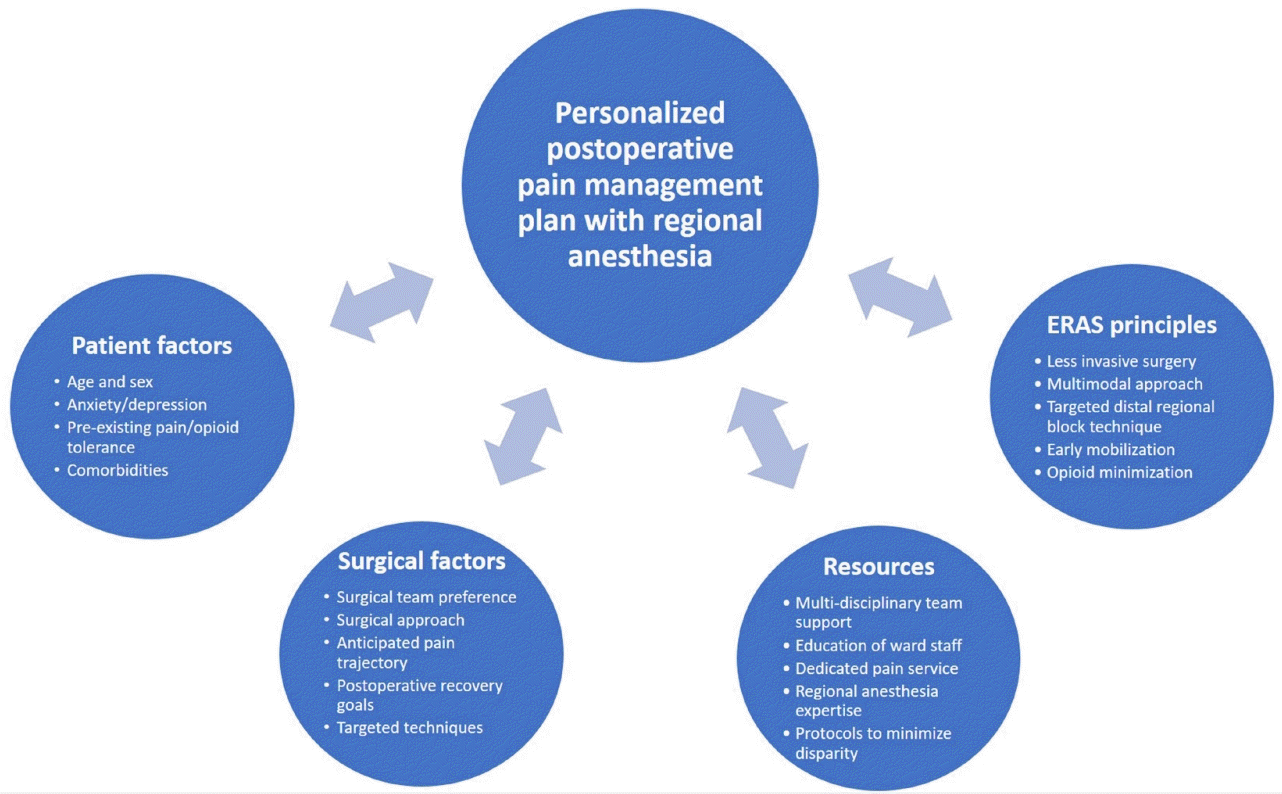
Consideration of patient and procedural factors, combined with the resources available at the center at which they practice, will allow anesthesiologists to formulate the most appropriate postoperative pain management plan, incorporating regional anesthesia techniques (Fig. 2). This is a process that should be considered by all anesthesiologists, not just regional anesthesia enthusiasts, for every patient in accordance with postoperative pain management guidelines [2] to effectuate ERAS principles.
Neuromodulation, typically used in chronic pain, may be a non-pharmacological option for acute postoperative pain that lasts for several days to weeks. Ilfeld and colleagues [49–51] have used this technique to provide analgesia following various types of surgery, including anterior cruciate ligament reconstruction, foot surgery, and TKA. Under ultrasound guidance, a lead is placed in a manner similar to perineural catheter insertion in proximity to a target nerve. Electrical current emitted from the indwelling lead is responsible for the subsequent pain control [49]. Although it may be more costly, neuromodulation is an alternative to cPNB that requires no infusate solution, produces no motor block, and can be maintained for up to two months [52]. Long-term outcome studies of this intervention, particularly on the incidence of PPSP and chronic opioid use, will be of great public health interest.
More research is required so that novel blocks can be incorporated into patient care pathways. Robust data are available for well-established regional analgesic techniques such as neuraxial and major nerve and plexus blocks. However, there are limited outcomes data for novel techniques such as fascial plane blocks. There are still many unanswered questions regarding the mechanism of action of fascial plane blocks [53], which may explain the variation in the clinical outcomes that have been reported. A major drawback is the heterogeneity of small datasets with varied techniques and outcome measures that do not allow for easy comparison and pooling of data. Establishment of standardized, clinically-relevant, and patient-oriented outcome measures may be the first step to improving the evidence for these novel techniques.
Precision medicine incorporating artificial intelligence may be a game-changer and has many potential applications for pain management [54]. Electronic health records, despite their shortcomings, provide large datasets that have the potential to inform medical decision making and improve patient care [55]. Neural networks may help to identify factors that predispose patients to experience greater than expected pain after surgery and predict pain trajectories. The future of perioperative pain management may be a personalized plan based on the patient's surgery, medical history, current medications, socioeconomic and demographic factors, baseline testing, pharmacogenetics, and trajectory modeling. Such an approach will allow for advanced preoperative planning and provide patients with a truly patient-centered experience.
ACKNOWLEDGMENTS
This manuscript is the result of work supported with the resources and facilities at the Veterans Affairs (VA) Palo Alto Health Care System (Palo Alto, CA, USA). The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Dr. Tang is a paid consultant for Clarius Mobile Health (Burnaby, BC, Canada).
Notes
Author Contributions
Shruti S. Chitnis (Conceptualization; Project administration; Visualization; Writing – original draft; Writing – review & editing)
Raymond Tang (Conceptualization; Methodology; Supervision; Writing – original draft; Writing – review & editing)
Edward R. Mariano (Conceptualization; Methodology; Project administration; Resources; Software; Visualization; Writing – original draft; Writing – review & editing)
References
1. Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017; 10:2287–98.

2. Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a clinical practice guideline from the american pain society, the american society of regional anesthesia and pain medicine, and the american society of anesthesiologists' committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016; 17:131–57.

3. Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of postsurgical pain: results from a US national survey. Curr Med Res Opin. 2014; 30:149–60.

5. Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North Am. 2005; 23:21–36.

6. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006; 367:1618–25.

7. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019; 393:1537–46.

8. Kehlet H. Postoperative pain, analgesia, and recovery-bedfellows that cannot be ignored. Pain. 2018; 159 Suppl 1:S11–6.

10. Desai N, El-Boghdadly K, Albrecht E. Epidural vs. transversus abdominis plane block for abdominal surgery- a systematic review, meta-analysis and trial sequential analysis. Anaesthesia. 2020; Advance Access published on May 8, 2020. doi: 10.1111/anae.15068.
12. Joshi GP, Kehlet H. Postoperative pain management in the era of ERAS: an overview. Best Pract Res Clin Anaesthesiol. 2019; 33:259–67.

13. Kandarian BS, Elkassabany NM, Tamboli M, Mariano ER. Updates on multimodal analgesia and regional anesthesia for total knee arthroplasty patients. Best Pract Res Clin Anaesthesiol. 2019; 33:111–23.

14. Kumar K, Kirksey MA, Duong S, Wu CL. A review of opioid-sparing modalities in perioperative pain management: methods to decrease opioid use postoperatively. Anesth Analg. 2017; 125:1749–60.
15. Mariano ER, Schatman ME. A commonsense patient-centered approach to multimodal analgesia within surgical enhanced recovery protocols. J Pain Res. 2019; 12:3461–6.
16. Long DR, Lihn AL, Friedrich S, Scheffenbichler FT, Safavi KC, Burns SM, et al. Association between intraoperative opioid administration and 30-day readmission: a pre-specified analysis of registry data from a healthcare network in New England. Br J Anaesth. 2018; 120:1090–102.

17. Echeverria-Villalobos M, Stoicea N, Todeschini AB, Fiorda-Diaz J, Uribe AA, Weaver T, et al. Enhanced recovery after surgery (ERAS): a prospective review of postoperative pain management under ERAS pathways and its role on opioid crisis in the United States. Clin J Pain. 2020; 36:219–26.
18. Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017; 152:691–7.
19. Richman JM, Liu SS, Courpas G, Wong R, Rowlingson AJ, McGready J, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006; 102:248–57.

20. Albrecht E, Chin KJ. Advances in regional anaesthesia and acute pain management: a narrative review. Anaesthesia. 2020; 75 Suppl 1:e101–10.

21. El-Boghdadly K, Wiles MD. Regional anaesthesia for rib fractures: too many choices, too little evidence. Anaesthesia. 2019; 74:564–8.

22. Turbitt LR, Mariano ER, El-Boghdadly K. Future directions in regional anaesthesia: not just for the cognoscenti. Anaesthesia. 2020; 75:293–7.

23. Chapman CR, Vierck CJ. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain. 2017; 18:359. e1-359.e38.

24. Yang MMH, Hartley RL, Leung AA, Ronksley PE, Jetté N, Casha S, et al. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open. 2019; 9:e025091.

25. Coppes OJM, Yong RJ, Kaye AD, Urman RD. Patient and surgery-related predictors of acute postoperative pain. Curr Pain Headache Rep. 2020; 24:12.

26. Sommer M, de Rijke JM, van Kleef M, Kessels AG, Peters ML, Geurts JW, et al. Predictors of acute postoperative pain after elective surgery. Clin J Pain. 2010; 26:87–94.

27. Weinrib AZ, Azam MA, Birnie KA, Burns LC, Clarke H, Katz J. The psychology of chronic postsurgical pain: new frontiers in risk factor identification, prevention and management. Br J Pain. 2017; 11:169–77.

28. Thomazeau J, Rouquette A, Martinez V, Rabuel C, Prince N, Laplanche JL, et al. Acute pain factors predictive of postoperative pain and opioid requirement in multimodal analgesia following knee replacement. Eur J Pain. 2016; 20:822–32.

29. Gramke HF, de Rijke JM, van Kleef M, Kessels AG, Peters ML, Sommer M, et al. Predictive factors of postoperative pain after day-case surgery. Clin J Pain. 2009; 25:455–60.

30. Safa R, Sadovnikoff N. Anesth Clin J Pain 2009; 25: 455-60esia for patients with concomitant cardiac and renal dysfunction. Anesthesiol Clin. 2016; 34:697–710.
32. Auyong DB, Hanson NA, Joseph RS, Schmidt BE, Slee AE, Yuan SC. Comparison of anterior suprascapular, supraclavicular, and interscalene nerve block approaches for major outpatient arthroscopic shoulder surgery: a randomized, double-blind, noninferiority trial. Anesthesiology. 2018; 129:47–57.
33. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: american society of regional anesthesia and pain medicine evidence-based guidelines (fourth edition). Reg Anesth Pain Med. 2018; 43:263–309.

34. Mariano ER, El-Boghdadly K, Ilfeld BM. Using postoperative pain trajectories to define the role of regional analgesia in personalised pain medicine. Anaesthesia. 2020; Advance Access published on May 5, 2020. doi: 10.1111/anae.15067.

35. Chung F, Ritchie E, Su J. Postoperative pain in ambulatory surgery. Anesth Analg. 1997; 85:808–16.

36. Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a cochrane systematic review and meta-analysis. Br J Anaesth. 2013; 111:711–20.
37. Prabhakar A, Lambert T, Kaye RJ, Gaignard SM, Ragusa J, Wheat S, et al. Adjuvants in clinical regional anesthesia practice: a comprehensive review. Best Pract Res Clin Anaesthesiol. 2019; 33:415–23.

38. Joshi G, Gandhi K, Shah N, Gadsden J, Corman SL. Peripheral nerve blocks in the management of postoperative pain: challenges and opportunities. J Clin Anesth. 2016; 35:524–9.

39. Uskova A, O'Connor JE. Liposomal bupivacaine for regional anesthesia. Curr Opin Anaesthesiol. 2015; 28:593–7.

40. Harky A, Clarke CG, Kar A, Bashir M. Epidural analgesia versus paravertebral block in video-assisted thoracoscopic surgery. Interact Cardiovasc Thorac Surg. 2019; 28:404–6.

41. Kosinski S, Fryzlewicz E, Wilkojc M, Cmiel A, Zielinski M. Comparison of continuous epidural block and continuous paravertebral block in postoperative analgaesia after video-assisted thoracoscopic surgery lobectomy: a randomised, non-inferiority trial. Anaesthesiol Intensive Ther. 2016; 48:280–7.
42. Scarci M, Joshi A, Attia R. In patients undergoing thoracic surgery is paravertebral block as effective as epidural analgesia for pain management? Interact Cardiovasc Thorac Surg. 2010; 10:92–6.

43. Mar GJ, Barrington MJ, McGuirk BR. Acute compartment syndrome of the lower limb and the effect of postoperative analgesia on diagnosis. Br J Anaesth. 2009; 102:3–11.

44. Mariano ER. Making it work: setting up a regional anesthesia program that provides value. Anesthesiol Clin. 2008; 26:681–92.

45. Hunter OO, Kim TE, Mariano ER, Harrison TK. Care of the patient with a peripheral nerve block. J Perianesth Nurs. 2019; 34:16–26.

46. Hernandez-Boussard T, Graham LA, Desai K, Wahl TS, Aucoin E, Richman JS, et al. The fifth vital sign: postoperative pain predicts 30-day readmissions and subsequent emergency department visits. Ann Surg. 2017; 266:516–24.
47. Mudumbai SC, Auyong DB, Memtsoudis SG, Mariano ER. A pragmatic approach to evaluating new techniques in regional anesthesia and acute pain medicine. Pain Manag. 2018; 8:475–85.

48. Swenson JD, Davis JJ. Getting the best value for consumable supplies in regional anesthesia. Int Anesthesiol Clin. 2011; 49:94–103.

49. Ilfeld BM, Said ET, Finneran JJT, Sztain JF, Abramson WB, Gabriel RA, et al. Ultrasound-guided percutaneous peripheral nerve stimulation: neuromodulation of the femoral nerve for postoperative analgesia following ambulatory anterior cruciate ligament reconstruction: a proof of concept study. Neuromodulation. 2019; 22:621–9.

50. Ilfeld BM, Gabriel RA, Said ET, Monahan AM, Sztain JF, Abramson WB, et al. Ultrasound-guided percutaneous peripheral nerve stimulation: neuromodulation of the sciatic nerve for postoperative analgesia following ambulatory foot surgery, a proof-of-concept study. Reg Anesth Pain Med. 2018; 43:580–9.
51. Ilfeld BM, Ball ST, Gabriel RA, Sztain JF, Monahan AM, Abramson WB, et al. A feasibility study of percutaneous peripheral nerve stimulation for the treatment of postoperative pain following total knee arthroplasty. Neuromodulation. 2019; 22:653–60.

52. Gabriel RA, Swisher MW, Ilfeld BM. Percutaneous peripheral nerve stimulation for acute postoperative pain. Pain Manag. 2019; 9:347–54.

53. Elsharkawy H, Pawa A, Mariano ER. Interfascial plane blocks: back to basics. Reg Anesth Pain Med. 2018; 43:341–6.

Fig. 1.
The mean worst pain scores for the following four surgical procedures: knee arthroplasty, hip arthroplasty, mastectomy, and shoulder arthroscopy (adapted from Mariano et al. [34]). * Data for the shoulder arthroscopy patients were only collected through postoperative day 3.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download