This article has been
cited by other articles in ScienceCentral.
Abstract
Background
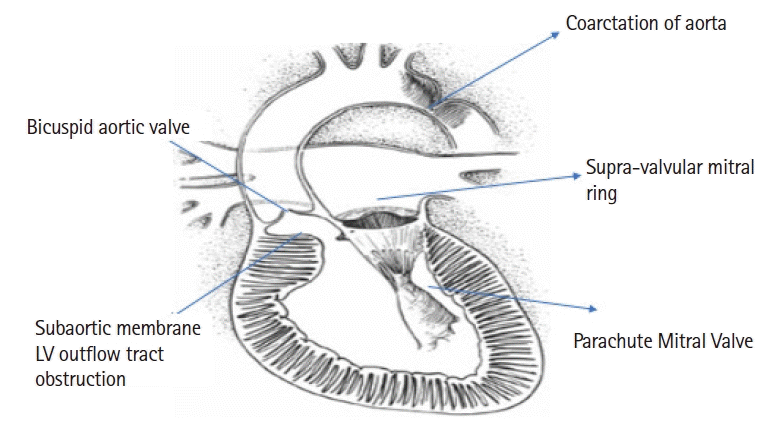
Shone’s syndrome is a rare complex congenital cardiac condition, characterized by a supra-valvular mitral ring, parachute deformity of the mitral valve, aortic stenosis, and coarctation of the aorta.
Case
A 26-year-old parturient with partial Shone’s syndrome presented to our delivery unit in pulmonary edema. She underwent a scheduled cesarean section performed under a combined spinal-epidural anesthetic at 33 weeks. She had multidisciplinary input from the cardiac, obstetric, and anesthetic teams, which led to a good outcome. A review of the five published case reports of Shone’s syndrome in pregnancy is presented along with key findings.
Conclusions
Our case report and the review highlight the successful use of combined spinal-epidural anesthetic and provides guidance to the multidisciplinary team on the varied presentation and the optimum management of women with Shone’s syndrome during the peripartum period.
Keywords: Aortic coarctation, Aortic stenosis, Cesarean section, Epidural anesthesia, Mitral valve stenosis, Spinal anesthesia
Heart disease remains one of the most common causes of maternal mortality. Shone’s syndrome (SS) was first described in 1963 by John Shone – a pediatric cardiologist [
1]. It is characterized by:
1) Left ventricular (LV) inflow tract obstruction in the form of a supra-valvular mitral valve (MV) ring or a parachute MV
2) LV outflow tract obstruction in the form of aortic stenosis (AS), which may be supra-valvular, valvular with a bicuspid aortic valve (BAV), or sub-valvular
3) Aortic abnormalities in the form of hypoplasia of the aortic arch or coarctation of aorta (Co-A).
The lesions encountered in SS are represented in
Fig. 1.
It has an incidence of 0.67% in adults with congenital heart disease and the most common lesions seen in this syndrome are congenital MV stenosis (93%), Co-A (75%), and AS (71%) [
2]. It can exist in a complete form (all lesions present), or more frequently in a partial or incomplete form (LV inflow obstruction and any one of the other abnormalities) [
2]. The syndrome is extremely rare in pregnancy. We describe our anesthetic management for a parturient with repaired but with residual SS who underwent an uneventful Cesarean section (C-section).
Case Report
Written informed consent was obtained from the patient. A 26-year-old primigravida with a body mass index (BMI) of 25.71 kg/m2 (weight 70 kg, height 165 cm) presented to our delivery suite in Manchester at 32-weeks with a 2-week history of dyspnea on exertion, orthopnea, and palpitations. She was known to have SS. Her syndrome consisted of a supra-valvular mitral ring, a parachute MV, mild LV outflow tract obstruction with Co-A, and a BAV. The mitral ring, LV outflow tract obstruction, and the Co-A were repaired at the age of three by open heart surgery, which was followed by a dual chamber pacemaker insertion for complete heart block. The BAV and the parachute MV were not repaired. This was followed by balloon dilatation of the aorta for re-coarctation at the age of 12. She remained asymptomatic following this till the end of the second trimester of pregnancy.
On presentation to our unit, she had a heart rate (HR) of 100 beats/min, blood pressure (BP) of 136/88 mmHg, respiratory rate (RR) of 28 breaths/min with oxygen saturations (SpO2) 90–92% on room air. Auscultation revealed bilateral crepitations along with a mid-diastolic murmur and a diagnosis of acute pulmonary edema was made. Arterial blood gas (ABG) revealed a pH – 7.48, partial pressure of carbon dioxide (pCO2) – 23.5 mmHg, partial pressure of oxygen (pO2) – 90.76 mmHg, base excess of 4 mmol/L with a lactate of 3 mmol/L. Her hemoglobin was 12.2 g/dl, serum potassium (K+) was 3.6 mmol/L, and serum magnesium (Mg+) was 0.65 mmol/L. Her N-terminal pro-B-type natriuretic peptide (NT-pro BNP) levels were 1,020 pg/ml. Her chest X-ray was suggestive of pulmonary edema and electrocardiogram (ECG) showed a sinus tachycardia with P mitrale.
She was transferred to the coronary care unit and the treatment instituted included oxygen, intravenous (IV) furosemide 20 mg, 5 mg of IV diamorphine, and oral bisoprolol 2.5 mg. Fluid balance was monitored using strict input and output monitoring with a urinary catheter, and oral potassium chloride and IV magnesium were supplemented to maintain K+ > 4 mmol/L and Mg+2 > 0.7 mmol/L.
Pacemaker check revealed an appropriately functioning dual chamber DDD device. Cardiotocography as part of fetal monitoring revealed a normal trace. A trans-thoracic echocardiogram (TTE) revealed:
1) A BAV with mild AS with a velocity of 2.5 m/s across the valve and a mean gradient of 25 mmHg
2) A normal LV size (LV diastolic diameter of 4.4 cm), with an ejection fraction of 48%, with mildly impaired systolic function
3) Parachute MV with chordal attachment to single papillary muscle, MV area of 1.12 m2, mean gradient of 8 mmHg across MV, moderate MV stenosis with mild-moderate regurgitation
4) Severely dilated left atrium (LA-volume/body surface area of 49 ml/m2)
5) A normal aortic root, with a normally functioning repair (velocity across the aorta was 2.4 m/s) with no diastolic tail
6) A mildly dilated well-functioning right ventricle (base RV of 4.3 cm and mid RV of 3.8 cm with preserved RV fractional area of change > 40%), mild tricuspid regurgitation (vena contracta width of 3 mm, max velocity of 3.4 m/s) with estimated mean pulmonary artery (PA) pressure of 56 mmHg with a moderately dilated right atrial volume of 67 ml.
Her case was discussed at the multi-disciplinary cardiology, obstetric, anesthetic team meeting. In view of her on-going symptoms, limited mobility, and detection of a severely dilated LA and raised PA pressure on TTE, the team decided to administer tinzaparin 4500 IU subcutaneously for thromboprophylaxis.
Despite medical management for the next 72 h, she complained of dyspnea on minimal exertion. She required 2 L of oxygen to maintain saturations of 97% (94% on air) but was able to lie almost flat without significant difficulty. A repeat ABG on oxygen, revealed a pH – 7.42, pCO2 – 30.5 mmHg, pO2 – 93.33 mmHg, base excess of 2 mmol/L with a lactate of 1.6 mmol/L. As thromboembolism remains one of the most common direct causes of death in pregnancy in the United Kingdom (UK), on a risk-benefit basis, based on her symptoms and dependence on oxygen, the team decided to rule out pulmonary embolism (PE) with a computerized tomography with pulmonary angiography (CTPA), which was reported back as normal.
In view of her symptoms and significant pulmonary hypertension, a decision was made to deliver her by category (Cat) 3 C-section in accordance with The Royal College of Obstetricians and Gynaecologists’ guidelines for classification of urgency of C-section (Cat 1: Immediate threat to life of woman or fetus, Cat 2: Maternal or fetal compromise, which is not immediately life-threatening, Cat 3: Needing early delivery but no maternal or fetal compromise, Cat 4: At a time to suit the woman and maternity team). Maternal steroids were administered to accelerate fetal lung maturity. After discussion with the patient of the potential risks and benefits of general anesthesia (GA) compared with neuraxial anesthesia, it was decided to perform the surgery under combined spinal-epidural (CSE) anesthesia. The decision process incorporated the patient’s preference to stay awake and witness the delivery of her baby along with partner in the operating theatre.
In theatre, a 16 gauge (G) peripheral cannula was inserted and the patient had ECG, SpO
2, and invasive BP monitoring was established in theatre via a radial artery catheter. Baseline HR was 86 beats/min and BP was 100/58 mmHg. A 12-h interval between the last dose of prophylactic tinzaparin and administration of CSE anesthetic was followed in accordance with The European Society of Anesthesiology guidance [
3]. CTG was monitored during and after the CSE insertion and was normal at all times.
With the patient in the sitting position, using an aseptic technique, the epidural space initially was detected with a 16 G Tuohy needle using a loss-of-resistance to saline technique at the L3–4 intervertebral space and an epidural catheter threaded into the epidural space. A test dose of 5 ml of 0.1% bupivacaine was given to rule out intrathecal catheter placement. This was followed by a subarachnoid injection at L4–5 interspace of 7.5 mg of hyperbaric bupivacaine and 300 ug diamorphine with a 25 G pencil point needle. The patient was positioned supine with left uterine displacement and 500 ml of compound sodium lactate (CSL) solution commenced along with a phenylephrine infusion 100 μg/ml at the rate of 30 ml/h. Within 12 min, a bilateral block to cold up to T8 to S5 dermatomes was established. To augment the block height, 5 ml of 0.75% ropivacaine was administered via the epidural catheter. Once the block height to T4 dermatome with cold spray was confirmed, C-section was commenced, and a female infant weighing 2.3 kg was delivered. APGAR scores of 5 at 1 min and 8 at 5 min were recorded. Oxytocin 5 IU was given as an IV infusion over 20 min to avoid tachycardia and hypotension. Pacemaker was kept on throughout the C-section. Patient was hemodynamically stable throughout, blood loss recorded during the C-section was 700 ml, and the procedure was completed uneventfully in 45 min. The phenylephrine infusion was weaned off gradually. Her epidural was removed at the end of the surgery to facilitate thromboprophylaxis following C-section. Tinzaparin 4,500 IU was administered subcutaneously 4 h after removal of epidural and continued for 10 days post-operatively. She stayed in our cardiac intensive care unit for 24 h, then stepped down to our obstetric high dependency unit over the next 48 h, and was discharged uneventfully from the hospital on the 7th post-operative day.
Discussion
To our knowledge, this is one of the first case report, highlighting the successful use of CSE in a parturient with SS. Considering that SS is a fixed cardiac output lesion, the physiological changes of pregnancy, including a 25% increase in HR, a 25% drop in systemic vascular resistance (SVR), a 40% increase in cardiac output, anemia, and a 25% increase in oxygen demand are poorly tolerated [
4]. Dyspnea on exertion, orthopnea, palpitations, and pulmonary edema are common presentations in SS when the MV stenosis is prominent, suggestive of heart failure or new onset arrhythmia. These were seen at 32 weeks in our parturient when the cardiac output peaks in pregnancy. On echocardiography, our patient had moderate MV stenosis, mild AS with a BAV, pulmonary hypertension, and a normal aorta. Appropriate medical therapy was instituted, and we ruled out PE with a CTPA in view of our patient’s persistent dyspnea.
The obstetric, cardiology, anesthetic as well as the neonatal teams were involved early in our case so as to plan the mode of delivery, analgesia, anesthesia, and post-partum care in accordance with the National Institute of Health Care and Excellence and the European Society of Cardiology guidelines [
5,
6]. Our team made the decision of delivering our patient with a C-section at 33 weeks in view of her persistent dyspnea, oxygen requirements, and significant pulmonary hypertension.
The optimal choice of analgesic and anesthetic technique for delivery in a patient with SS where MV stenosis as well as AS is predominant lesion should encompass the following goals:
1) Optimum analgesia
2) A slow HR to decrease oxygen demand and increase diastolic filling time
3) Maintain sinus rhythm
4) Avoiding fall in SVR and maintaining contractility
5) Avoiding any increase in pulmonary vascular resistance (PVR), (hypoxia, hypercarbia, acidosis, hypothermia, high positive end-expiratory pressure)
6) Avoidance of Valsalva maneuver and shortening the second stage of labor
7) Avoiding fluid overload, aortocaval compression, and maintaining euvolemic status and if coarctation exists, avoid swings in BP and hypertension.
Neuraxial anesthesia for C-section in fixed cardiac output lesions though may result in a drop in SVR, but when titrated appropriately with a suitable vasopressor, might be a technique of choice in these cases and has been reported in AS and MV stenosis [
7,
8]. We opted for a CSE technique as it allowed us to place a small intrathecal dose of local anesthetic along with an opioid to initiate the block, the final height of which could then be titrated using the epidural top up. The low dose of local anesthetic in CSE provided us with a good quality of block, avoided the sudden hypotension, and the intrathecal diamorphine added to the local anesthetic contributed to good post-operative pain relief. A CSE technique with separate needle and separate interspaces was utilized in our case as it allowed us to test the epidural catheter before placement of intrathecal drug. After intrathecal local anesthetic with opioid achieved a block of T8, we topped up our epidural catheter with ropivacaine to augment block height to T4 dermatome, which allowed the surgery to be carried out uneventfully. BP was maintained in our case by using phenylephrine infusion, which is the vasopressor of choice in obstetric anesthesia. It also avoids tachycardia and maintains SVR, which was advantageous in SS.
Other options for neuraxial anesthesia include using:
1) A de novo spinal anesthetic (SA), which could lead to a dramatic drop in SVR with an unpredictable spread
2) A continuous SA using an intrathecal catheter, which we were unfamiliar with
3) A de novo epidural technique, which is associated with incomplete sensory and motor block, and conversion to GA.
We avoided a GA as our patient was keen to stay awake during the C-section. GA has the advantage of secure airway and the ability to perform a real time transesophageal echocardiography but also has the disadvantages of sympathetic stimulation associated with laryngoscopy, positive pressure ventilation, and increasing PVR thus decreasing venous return, as well as the known obstetric risks of difficult intubation, aspiration, and awareness.
We chose IBP monitoring in our case using an arterial line to facilitate beat-to-beat BP monitoring and blood gas analysis as our patient was dependent on oxygen. Central venous access was not thought to be necessary as we were mindful of the possible risk of inducing an arrhythmia. Oxytocin was given as a slow infusion to avoid tachycardia and hypotension and fluid neutral balance was maintained replacing blood loss with CSL. Though our neonate was premature, a good neonatal outcome was reported in our case.
We present a systematic review of all the published case reports of SS over the last two decades. Using the NICE Healthcare Databases’ advanced search engine, a search of the Medline, CINAHL, and EMBASE databases from January 1, 2000 to December 31, 2019 was conducted in January 2020. The following search terms were used in the search strategy: Shone’s syndrome OR Shone’s complex OR Shone’s anomaly AND Obstetric OR Pregnant OR Labor OR Cesarean. The search was limited to humans, and to case reports written and published in English. All articles generated had their reference lists and citations hand-checked by the authors and any additional articles were scrutinized. Full text articles were included in the analysis if they confirmed the diagnosis of SS and described the mode of delivery. Information extracted from the case reports included age, parity, BMI, mode of delivery, weeks of gestation, clinical presentation, echocardiographic findings, anesthesia details, and neonatal outcomes.
Results
We found five published case reports of SS in pregnancy since the year 2000 [
9–
13]. Their demographics, initial presentation to the delivery unit, echocardiogram findings, maternal, and neonatal outcomes along with their anesthetic management are presented in
Table 1.
Mean age of women in the literature review was 22.6 years, mean BMI was 26.3 kg/m
2, and three of the five women (60%) were primiparous [
10,
11,
13]. All the published case reports in our review had a partial or incomplete form of SS (100%) with two of the five women (40 %) having some form of surgical correction in childhood [
12,
13].
Clinical presentation
Dyspnea on exertion, orthopnea, palpitations, and pulmonary edema were the presenting symptoms in three case reports (60%) [
10,
11,
13]. Beta blockers and diuretics were commonly utilized in these cases.
Women can also present with systemic hypertension where Co-A features in SS prominently. In three of the case reports (60%), the reported BP on presentation was greater than 140/90 mmHg [
9,
10,
13]. These patients may be mistakenly diagnosed with pre-eclampsia but absence of proteinuria, a normal urinary:protein creatinine ratio, and use of biomarkers such as the ratio of sFlt-1 (soluble FMS-like tyrosine kinase-1; an anti-angiogenic factor)/PlGF (placental growth factor; an angiogenic factor) might provide clues to the obstetric team in ruling out pre-eclampsia [
14].
Echocardiography findings
In the five case reports described in our review:
1) MV: Parachute MV was seen in one woman (20%) [12]. Dysplastic MV leaflets were observed in one case (20%) and mild MV stenosis seen in one (20%) woman [9,11]. Supra-valvular mitral ring was seen in one of the women (20%) [10]. Valve area varied between 1.2 and 2.17 m2 and gradient across MV varied between 14 and 22 mmHg [10,12,13]. Overall, MV stenosis was found in three of the five case reports (60%).
2) Aortic valve: BAV with AS was seen in three of the five case reports (60%). Three women had AS with peak gradients varying from 35 to 80 mmHg with one patient having mild AS [9,11,13]. Subaortic membrane was resected (40%) in two cases [12,13] with the aortic valve being normal (40%) in two [10,12].
3) Co-A: Two of the women had Co-A at the time of C-section (40%) with gradients varying from 37 to 70 mmHg [10,13]. One woman had proximal aortic aneurysm (20%) and one had a normal (20%) aorta [9,11]. Two of the women had Co-A repaired (40%) during childhood [12,13].
The echocardiographic findings in the review highlight that MV stenosis along with BAV seems to be the most common findings in SS in pregnancy. It is important that if MV stenosis of non-rheumatic origin or a parachute MV is noted on the echocardiogram, the cardiology team should look out for other lesions to confirm the diagnosis of SS.
Multidisciplinary input
Of the five case reports two of them (40%) had multidisciplinary input in them [
12,
13]. Multidisciplinary input by the obstetric cardiac team is recommended for heart disease in pregnancy as per the European Society of Cardiology guidance [
6].
Mode of delivery
This will be dictated by a number of factors including both obstetric as well as cardiac. In women with severe cardiac lesions, significant pulmonary hypertension, heart failure, and a dilated aortic root, a C-section might be the preferable mode in line with the European Society of Cardiology recommendations [
6]. Vaginal delivery (20%) was reported in just one of the case reports with SS [
10]. C-section (80%) was reported in four other cases [
9,
11–
13].
Anesthesia and analgesia
Three of the five cases (60%) having C-section described the anesthetic management in detail [
9,
11,
13]. Use of de novo epidural anesthesia was reported in two cases [
11,
13]. One proceeded uneventfully; the other case resulted in significant hypotension, fetal distress, and a conversion to an unplanned GA [
11]. Thiopentone and suxamethonium were utilized in that case. Planned GA was administered using etomidate, propofol target-controlled infusion, remifentanil, and rocuronium in one case to manage a SS lady who also had an ascending aortic aneurysm, which was being repaired at the time of CS [
9]. No analgesia was utilized in the case of the woman having a vaginal delivery [
10]. If choosing a GA technique, a rocuronium-sugammadex combination for muscle relaxant and a reversal might avoid the tachycardia seen with glycopyrrolate and neostigmine and might be advantageous in this cohort.
Blood pressure management and monitoring
Phenylephrine was used in two (40%) of the five case reports [
11,
13]. Ephedrine should be avoided in this cohort. Arterial line monitoring was utilized in three (60%) of the five reports [
9,
10,
13]. Central venous access was utilized in two (40%) of the case reports [
9,
13]. Though fluid was administered in one case report using the central venous pressure (CVP) monitor, we are unsure in a patient with valvular stenosis how reliable CVP monitoring would be to guide fluid replacement.
In women presenting with active Co-A, the post ductal BP is more suggestive of uterine perfusion. BP should be maintained to avoid compromising utero-placental blood flow and systemic hypertension [
15].
Oxytocic
Two of the five case reports (40%) describe the use of oxytocin infusion to maintain uterine tone [
9,
13]. Regarding oxytocic agents, it is best to avoid ergometrine in SS as it does cause hypertension with tachycardia and prostaglandin F2 alpha in the presence of pulmonary hypertension as it can increase PVR. The cardiac output does peak again post-delivery and these patients are at risk of pulmonary edema following delivery; hence fluid should be administered cautiously aiming for a neutral fluid balance.
Neonatal outcomes
Preterm (gestational age < 37 weeks) birth was reported in two of the five case reports (60%) as was seen in our case as well [
9,
13]. Mean gestational age at the time of delivery was 35.75 weeks on our review. Three of the other case reports described normal APGAR scores (60%) with mostly good outcomes [
10,
11,
13]. In one of the case reports where GA was administered, the neonate was intubated (20%) and ventilated [
9].
The information detailed in the review of literature could provide useful information for the obstetric cardiac team when preconception counseling and risk-assessment are undertaken in women with SS. Based on the review of the literature, we provide a summary of recommendations, which could be utilized by the multidisciplinary cardiac, obstetric, anesthetic, and neonatal team when they encounter a parturient with SS (
Table 2).
Limitations of our review include limited number of patients, some information that was missing in the case reports, and it being limited to only the last two decades. There is also a possibility that we might have missed case reports in other languages as the literature review was limited to case reports in English.
In conclusion, our case report along with the review of literature raises awareness about this condition, highlights the safe use of CSE anesthesia, and provides guidance to the multidisciplinary obstetric, cardiac, anesthetic, and neonatal team on the varied presentation and the optimum management of women with SS during the peripartum period.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download