Abstract
Background
Montgomery T-tube (MTT) is a useful airway device but is seldom used. Owing to its specific shape and structure, it is challenging for anesthesiologists to manage airway in patients with MTT in situ who require general anesthesia and continuous positive-pressure ventilation (CPPV).
Case
A 48-year-old man (weight 74 kg) with an MTT in situ was scheduled for local pancreatic resection under general anesthesia. We transorally inserted a cuffed endotracheal tube into the intratracheal limb of the MTT to achieve CPPV and to deliver the inhalation anesthetic. The endotracheal tube was removed successfully after the patient fully recovered from anesthesia. No tracheal injury or hemorrhage occurred after intubation or extubation, and the location of the MTT remained unchanged.
The use of a Montgomery T-tube (MTT) was first described in 1965 [1]. The MTT is employed as both a stent and a tracheal tube in patients with airway collapse, subglottic stenosis, and those undergoing laryngotracheal reconstruction [2]. It is a silicone T-tube that consists of an intratracheal limb placed into the trachea and an extratracheal limb passing through the tracheotomy stoma. The intratracheal limb is uncuffed, while the extratracheal limb has no standard connector for anesthetic circuit [1]. MTT is available in different sizes, with outer diameter (OD) ranging from 4.5 mm to 16 mm, which can be selected to fit the trachea in both adults and children [3]. Owing to its distinctive design and infrequent use, anesthesiologists have less experience managing the airways of patients with MTT in situ who require general anesthesia and continuous positive-pressure ventilation (CPPV). Herein, we describe a case of tube-in-tube airway management in a patient with MTT in situ to administer CPPV.
A 48-year-old man (weight 74 kg) who was diagnosed with necrotizing pancreatitis was scheduled for local pancreatic resection under general anesthesia. One year earlier, the patient developed acute pancreatitis and underwent mechanical ventilation by endotracheal intubation. After his discharge from the hospital for one month, a severe dyspnea happened, caused by subglottic stenosis. His tracheal stenosis was mostly attributed to the previous instance of prolonged intubation, mechanical ventilation, and cuff injury. Unfortunately, repeated balloon dilatation under bronchofibroscopy failed to cure his tracheal stenosis. Finally, a 12-mm sized MTT (Boston Medical Products, USA) was used to support his trachea. The West China Hospital Review Board approved the study (no. 2020-75) and informed consent was obtained.
Preoperatively, we intended to replace the MTT with a cuffed tracheostomy tube before anesthesia induction. However, the otorhinolaryngologist and respiratory physician who performed the tracheostomy and MTT insertion, respectively, forewarned of a high risk of tracheal hemorrhage or collapse after MTT removal, and that re-insertion of the MTT would be extremely difficult. Therefore, we decided to retain the MTT in situ in the trachea and prevent its displacement.
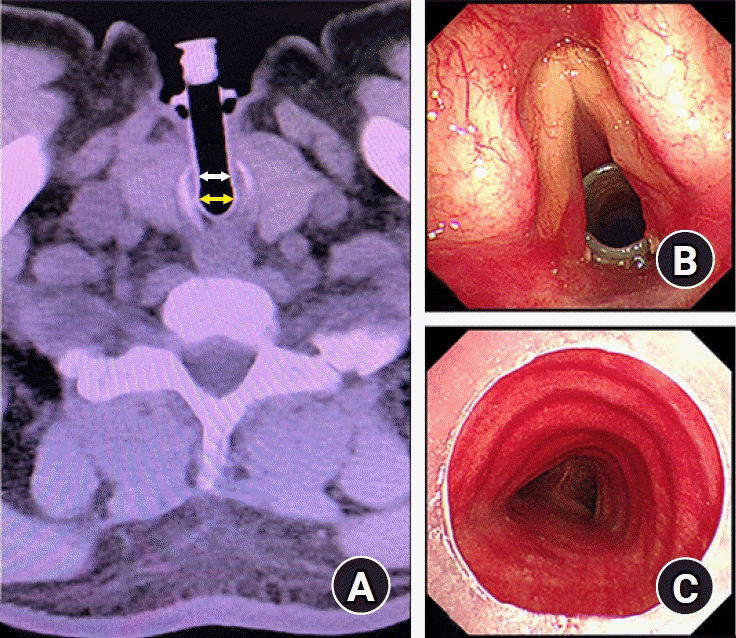
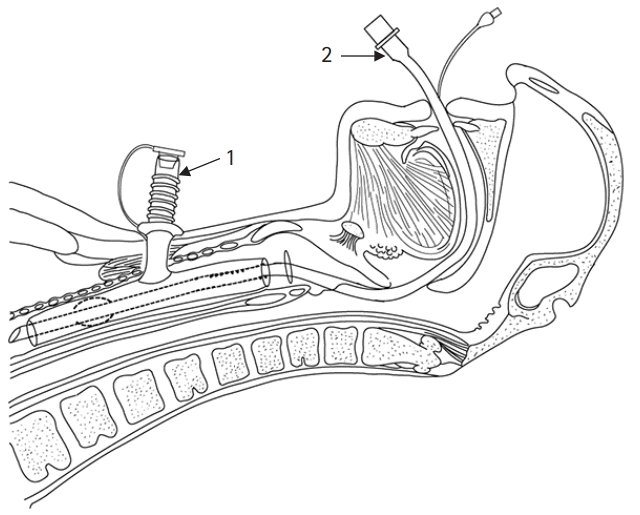
Based on the information provided by the manufacturer, the OD of the intratracheal and extratracheal limbs were 12 mm and 11 mm, respectively, and the lengths of the intratracheal and extratracheal limbs were 65 mm and 50 mm, respectively. Preoperative chest computed tomography (CT) scan and bronchofibroscopy examination showed that the lumens of the MTT were unobstructed. According to preoperative CT images, the internal diameters (ID) of the intratracheal and extratracheal limbs were estimated to be 10 mm and 8 mm, respectively (Fig. 1). We evaluated that either a size 6.5 (ID: 6.5 mm, OD: 8.9 mm) or size 6.0 (ID: 6.0 mm, OD: 8.2 mm) cuffed endotracheal tube (Covidien LLC, USA) could pass through the intratracheal limb, while simultaneously ensuring sufficient tidal volume. Finally, we planned for transoral insertion of an endotracheal tube through the MTT’s intratracheal limb to administer CPPV (Fig. 2).
The patient’s blood pressure, heart rate, pulse oxygen saturation, and bispectral index were monitored routinely. A well-lubricated video laryngoscope (E.An-II Plus, Tianjin Medan Medical Corp., China), cuffed endotracheal tubes (size 6.5 and 6.0), and a fiberoptic scope were prepared. First, the respiratory tract was cleaned by suction through the extratracheal limb of the MTT before anesthesia induction. Then, the patient inhaled 100% oxygen through a facemask that covered both his nose and mouth. Simultaneously, the extratracheal lumen was occluded. After 6 minutes of preoxygenation, 1% sevoflurane was inhaled through the facemask, and the sevoflurane concentration was gradually increased to 4%. When the patient was unconscious, but still breathing spontaneously, positive-pressure facemask ventilation was initiated, with no obvious gas leakage observed at the tracheostomy stoma. Thereafter, succinylcholine (100 mg) and propofol (100 mg) were intravenously administrated, and a video laryngoscope was immediately inserted into the mouth after the fasciculation disappeared. The glottis and the upper end of the intratracheal limb in the subglottis, without granulation tissue, were clearly visualized by the video laryngoscope. A size 6.5 cuffed endotracheal tube was inserted into the intratracheal lumen smoothly. To prevent MTT displacement, an assistant held the extratracheal limb lightly during intubation. Then, bronchofibroscopy was used to guide the distal end, and the cuff of the endotracheal tube passed entirely through the intratracheal limb and located above carina. The depth of the endotracheal tube was 23 cm. While the cuff was inflated, the endotracheal tube was connected to the anesthetic circuit for CPPV. An inspiratory pressure of 15 cmH2O achieved a 6–8 ml/kg of tidal volume. Clear and symmetrical breath sounds were confirmed without MTT displacement or tracheal hemorrhage. Then, sufentanil (20 μg) and cisatracurium (10 mg) were infused. Anesthesia was maintained using sevoflurane inhalation (1–2%), with continuous remifentanil infusion and intermittent administration of cisatracurium.
The operation lasted approximately 4 hours, and the respiratory tract was regularly cleaned through the endotracheal tube till the end of the surgery. After the patient fully recovered from anesthesia, extubation was performed under bronchofibroscopy, while holding the extratracheal limb. No tracheal injury or hemorrhage was detected, and the location of MTT remained unchanged.
MTT is usually used as a stent to maintain airway patency in patients with airway collapse, subglottic stenosis, or post-laryngotracheal reconstruction [2]. There were few cases of airway management in patients with MTT in situ who required general anesthesia and CPPV. Most anesthesiologists are unfamiliar with this irregular tracheal device.
Comprehensive preoperative assessment is very important for anesthesiologists to determine whether the pre-existing MTT could be kept while inducing general anesthesia. The common complications of MTT in situ are tracheal obstruction and re-stenosis, which are mostly induced by sticky secretions or granulation tissue at either ends of the MTT [2]. Because MTT itself is relatively soft, distortion occasionally occurs to limbs if it is compressed by local tracheal stenosis or cicatricial contracture. In these circumstances, the MTT may not be retained when patients are undergoing CPPV or induced with general anesthesia. If the shape and function of the MTT are normal, it could be considered to be reserved.
For patients with a normal MTT in situ, a laryngeal mask has been proposed for CPPV, with the occlusion of the extratracheal limb [3–5]. However, for patients with a high risk of aspiration, CPPV with laryngeal masks may not be a safe method for airway protection [3]. Connectors of endotracheal tubes have also been used to connect the extratracheal limb of the MTT with anesthetic circuit for mechanical ventilation, and with the upper lumen of intratracheal limb occluded simultaneously by a balloon catheter [3,4]. Because the connector is non-standard, it may not fit well with the MTT well, and the tightness and reliability of the connection cannot be ensured. An ill-matched connector may damage the wall of extratracheal limb. Additionally, no specialized balloon catheter is available for MTT.
It was also suggested that an endotracheal tube could be inserted from the extratracheal limb into the inferior lumen of the intratracheal limb before anesthesia induction [4]. The advantage of this method is that the airway may be protected before anesthesia induction because the procedure can be performed when the patient is conscious. However, for most MTTs, the ID of the extratracheal limb is smaller than that of the intratracheal limb, which will necessitate a much smaller endotracheal tube that can pass through it. In contrast, the intratracheal limb was almost perpendicular to the extratracheal limb, which made it difficult to insert an endotracheal tube through an angle with approximately 90 degree.
Placing a cuffed endotracheal tube transorally into the intratracheal limb may be regarded as a feasible and safe method. Because only the data of OD and length of each limb are provided by the manufacturer, it is very important for anesthesiologists to measure the ID of each limb. Occasionally, some irregular types of MTT are also used that have an intratracheal limb with tapered ends [4]. Thus, to select a suitable size of endotracheal tube that can pass entirely through the intratracheal limb of the MTT, the minimal ID of the intratracheal limb should be evaluated. Otherwise, a small endotracheal tube will induce high airway pressure or insufficient tidal volume, and a large endotracheal tube may cause repeated intubation or displacement of the MTT. CT examination is a useful method to obtain the minimal ID of each limb by measuring at different cross sections.
To improve success rate of first intubation or minimize tracheal injury, preparation of visual equipment and well-lubricated endotracheal tubes are prerequisite. In our case, muscle relaxation with succinylcholine might have aided the successful intubation with a relatively large endotracheal tube. Holding the extratracheal limb can decrease the risk of unexpected removal or displacement of the MTT both during intubation and extubation [3]. To occlude the trachea completely and to prevent gas leakage or damage to the MTT wall, the cuff of the endotracheal tube should entirely pass through the intratracheal limb under bronchofibroscopy guidance. Bronchofibroscopy examination should be performed both after intubation and extubation to detect any abnormalities of the trachea or MTT. Moreover, a skilled otorhinolaryngologist or respiratory physician should be present during intubation and extubation for emergency tracheostomy tube insertion or MTT re-insertion. The administration of anticholinergic agents in patients under these conditions may be controversial because, though it can reduce secretion, it may induce stickier secretions. Full suction is needed for cleaning the respiratory tract.
If MTT cannot be kept in situ due to severe tracheal obstruction or re-stenosis, it needs to be removed or replaced with a new one or a cuffed tracheostomy tube by respiratory physician or otorhinolaryngologist before anesthesia. Another solution is replacing the abnormal MTT with an endotracheal tube before anesthesia induction and then re-inserting a new MTT immediately after extubation by multi-discipline cooperation. However, the feasibility of this assumption needs further validation. A comprehensive preoperative assessment, multi-discipline cooperation, and adequate preparation are crucial for anesthetic planning in patients with MTT in situ. Transoral insertion of a suitable-sized cuffed endotracheal tube into the intratracheal limb could be considered a safe and feasible approach for the airway management in those patients who require CPPV.
ACKNOWLEDGMENTS
We are grateful to Chaozhi Luo and Chunling Jiang, who provided help in anesthetic management of this patient.
References
1. Montgomery WW. The surgical management of supraglottic and subglottic stenosis. Ann Otol Rhinol Laryngol. 1968; 77:534–46.
3. Wouters KM, Byreddy R, Gleeson M, Morley AP. New approach to anaesthetizing a patient at risk of pulmonary aspiration with a Montgomery T-tube in situ. Br J Anaesth. 2008; 101:354–7.

Fig. 1.
(A) Based on preoperative chest computed tomographic image, the internal diameters of the intratracheal limb (yellow arrow) and extratracheal limb (white arrow) were approximately 10 mm and 8 mm, respectively. Preoperative bronchofibroscopy examination showed (B) the superior end and (C) inferior end of the intratracheal limb were unobstructed without granulation tissue or sticky secretion.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download