Abstract
Background
Since the first case of severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) occurred in Wuhan in December 2019, the virus has spread globally. The World Health Organization declared the virus outbreak a pandemic on March 11, 2020. On January 19, 2020, a 35-year-old woman who returned from China was confirmed as the first SARS-CoV-2 infected case in Korea. Since then, it has spread all over Korea.
Case
We report the first case of a SARS-CoV-2 positive woman delivering a baby through cesarean section at 37+6 weeks of pregnancy in the Republic of Korea.
Conclusions
This case suggested that negative pressure operating room, skillful medical team, and enhanced personal protective equipment including N95 masks, surgical cap, double gown, double gloves, shoe covers, and powered air-purifying respirator are required at the hospital for safe delivery in such a case.
There are no published cases of cesarean section (C-sec) performed in pregnant women with severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2). Here, we report the first case of a woman delivering a baby through C-sec at 37+6 weeks of pregnancy in the Republic of Korea. Since the first case of Coronavirus disease (COVID-19) caused by SARS-CoV-2 occurred in Wuhan in December 2019, the virus has spread to all the regions of China [1]. The World Health Organization (WHO) announced SARS-CoV-2 as the cause of pneumonia on January 9, 2020, and declared the virus outbreak a pandemic on March 11, 2020.
On January 19, 2020, a 35-year-old woman who returned from China was confirmed as the first SARS-CoV-2 infected case in Korea. It has spread all over Korea since then, and the number of confirmed patients as of March 27, 2020 is 9,241. The mortality rate is approximately 0.91%. Currently, there are 6,482 confirmed patients in Daegu City alone [2].
Pregnant women are usually considered a high-risk group for viral infections, such as SARS-CoV-2, due to their lowered immune response and the effects of SARS on the fetus [3]. Moreover, delivery induces massive bleeding and loss of amniotic and body fluids, causing unstable vital signs. Although some pregnant women have been confirmed positive for SARS-CoV-2 in Korea, the impact of the virus on pregnant women has not yet been determined [4].
Our center has been designated for pregnant women infected with SARS-CoV-2 or those with symptoms caused by the virus, including fever, sore throat, cough, sputum, and pneumonia. Here, we report a case of anesthesia administered in a pregnant patient infected with SARS-CoV-2 and the protection of fetus and medical staff during the surgery.
Written informed consent was obtained from the patient.
A 28-year old pregnant woman (gravida 0, para 0) was recommended for C-sec at a local gynecology hospital due to cephalopelvic disproportion. She had been in contact with a SARS-CoV-2 infected patient, and she began self-isolation on February 14, 2020. She developed fever (> 38℃), mild sore throat, and cough, after which she visited a public health center and was tested positive for SARS-CoV-2 on February 25 (36+2 weeks gestation). She self-isolated herself at home after diagnosis and observed her prognosis. She had mild symptoms and received conservative treatment without medication. The symptoms improved and she only had a mild cough with no fever and sputum on February 29. She wanted to delay delivery until she recovered from the infection. However, an emergency C-sec was decided on March 6 (37+6 weeks) due to obstructed labor with incomplete rotation of the fetal head. The patient was transferred to our center, which is a designated hospital for pregnant patients infected with SARS-Cov-2. Her blood type was ‘O-Rh (+)’ and pre-natal workup did not show any specific findings. On arrival, she was immediately transferred to the radiology center for chest radiographs and computer tomography (CT) scans. We then transferred her to a pre-treatment room in the delivery center equipped with negative-pressure ventilation, exclusive elevators, and pre-operative laboratory test kits, including electrocardiograph (ECG), X-ray machine, blood tests, and urine test. Hemoglobin was 10.9 g/dl, erythrocyte sedimentation rate was slightly elevated (42 mm/h), C-reactive protein was normal (0.15 mg/dl), and other pre-operative laboratory test and ECG results seemed normal. The chest radiographs revealed left lower/middle lobe consolidation and increased vascular marking (Fig. 1A). We also observed multifocal peribronchial ground glass appearance and consolidation in the left lower lobe from chest CT scans (Fig. 1B). The SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) result of sputum and nasopharyngeal swab was obtained pre-operatively. Baseline fetal heart rate was 129 beats/min.
The C-sec was performed in an operating room at the delivery center, which is located on a different floor from the main operating room (Figs. 2A and 2B). Hence, the traffic line of the patient did not overlap with that of the other surgical patients. In this case, spinal anesthesia was selected as the anesthetic method, because even if we started with spinal anesthesia, there could still be chances of switching to general anesthesia in case of inadequate anesthesia or sudden changes in the patient’s clinical condition. We were already equipped with a ventilator protected by three mechanical high-efficiency particulate air filters, video laryngoscope, fiber-optic laryngoscope blades and handles, endotracheal tubes (6.5 mm, 7.0 mm, and stylet), medication drugs such as propofol, lidocaine, rocuronium, sugammadex, atropine, and closed suction catheter before the patient entered the operating room.
All medical staff members wore enhanced personal protective equipment (PPE), including N95 mask, surgical cap, double gown, double gloves, shoe covers, and a powered air-purifying respirator (Figs. 3A and 3B) in the fitting room (Fig. 2B). In the operating room, we measured the initial blood pressure (115/69 mmHg), heart rate (82 beats/min), and peripheral capillary oxygen saturation (99%) under a facial N95 mask without any O2 supply [5], and the ECG result showed normal sinus rhythm.
The patient was 166 cm in height and 62 kg in weight. She was placed in the left lateral decubitus position. Spinal anesthesia was performed with a 25-gauge Pencan spinal needle at the L3/4 interspace, and 9 mg of 0.5% marcaine and 20 μg of fentanyl were injected intrathecally. She was placed in the supine position with a left lateral tilt after 10 min, and anesthesia was assessed bilaterally with cold alcohol cotton and the T4 level blockage was checked.
After spinal injection, the patient experienced nausea and had low blood pressure (71/40 mmHg). The nausea improved with elevation in blood pressure (103/60 mmHg), and the vital signs stabilized after five injections of 100 μg phenylephrine and fast dropping 400 cc of Hartmann solution and 250 cc of colloid (Volulyte®, Fresenius Kabi, Bad Homburg, Germany). The C-sec was performed uneventfully. The baby was born 6 min after incision, and then 100 mg carbetocin was administered intravenously shortly after the birth. Oxytocin 20 IU/1000 ml Hartmann dextrose was also infused continuously to produce uterine contraction and minimize the blood loss.
Total anesthesia time was 50 min and operation time was 40 min (Table 1). An estimated blood loss of 400 cc was noted, and 780 cc of crystalloid and 250 cc of colloid were administered during surgery. For pain control, a patient-controlled analgesia pump (ANAPA AC0605®, Ehwa Biomedics, Korea) with butorphanol (10 mg), ketorolac tromethamine (180 mg), ramosetron (0.6 mg), and normal saline (26 ml) was used.
During the recovery state, the patient stayed in the operating room. Her vital signs remained stable and the patient had no complaints. Recovery blockage on T8 was confirmed and she was transferred to a single room in the SARS-CoV-2 ward (another SARS-CoV-2 suspected mother stayed separately there) through an exclusive elevator with a nurse wearing PPE.
Both SARS-CoV-2 RT-PCR tests conducted on March 6 and March 8 were confirmed negative. Therefore, the patient was transferred from the SARS-CoV-2 ward to the general ward on March 10 and discharged on March 11 without any complications.
The baby girl weighing 3130 gm was born on March 6, 2020 at 11:22 with Apgar scores at 1 and 5 min of 9 and 10, respectively. She was transferred immediately to a private newborn’s room in the neonatal intensive care unit (NICU) in order to avoid being exposed to SARS-CoV-2. The NICU consists of two separate spaces: one for suspected or confirmed SARS-CoV-2 babies and the other for healthy babies. All four newborn babies delivered from mothers with suspected SARS-CoV-2 tested negative and were transferred to the latter in the NICU.
The SARS-CoV-2 RT-PCR results using placenta, amniotic fluid, and cord blood were negative. Furthermore, the nasopharyngeal swab of the baby was negative on two consecutive SARS-CoV-2 RT-PCR tests. The medical staff had to wear level ‘D’ PPE until their SARS-CoV-2 PCR testing was reported negative.
The neonate was in a healthy state getting oral feeding and was discharged with her mother.
The impact of SARS- CoV-2, a highly infectious respiratory virus, on pregnant women has yet to be reported. We performed a C-sec in a SARS-CoV-2 pregnant patient for the first time in Korea.
Our center has established guidelines as per the recommendations for Middle-East respiratory syndrome-Coronavirus (MERS-CoV) and WHO [6,7], and we have performed emergency C-sec on such patients with an experienced medical team. Although COVID-19 has a reportedly lower mortality rate than MERS-CoV (3% and 40%, respectively), it shows a higher reproduction rate (1.4–5.5 and < 1, respectively) [8]. Therefore, we needed to be prepared more thoroughly when dealing with COVID-19 cases. We prevented the affected patient from being exposed to the virus from the hospital entrance by not passing through the emergency room or obstetric outpatient clinic. After performing chest radiographs and CT scans in a radiation room, we transferred her to the delivery center. The delivery center is located on a different floor from the main operating room and temporarily equipped with negative-pressure ventilation for this surgery. Although we did not have devices to measure the negative pressure level, we could confirm the negative pressure by airflow visualization test (smoke test) (Video 1) [6,9]. To check the negative pressure in the operating room, we held a smoke tube around 5 cm in front of the base of the closed door. If the room were under negative pressure, the smoke would flow into the room. We were careful to check that the generated smoke velocity did not overpower the air velocity [10].
Although the smoke test has an advantage of checking the flow of air intuitively, it does not check the continuous pressure in the room. To overcome this disadvantage, we hung a light plastic material that shook in the air in the space of the closed door to check the airflow continuously. After that, we ventilated the delivery room for more than 100 min after surgery.
Regional anesthesia is recommended because it is known to be safer for both mother and fetus than general anesthesia [11,12]. Chest radiographs showed increased vascular marking and consolidation in the left middle lobe and left lower lobe. The patient had previously presented with cough and sputum; hence, regional anesthesia was recommended because general anesthesia could cause unnecessary aerosol generation [13].
Anesthesia and surgery were performed by experts, which ensured reduced anesthesia time and unnecessary virus exposure time. Fortunately, the SARS-CoV-2 RT-PCR test of the baby was negative. This could be because the exposure of the virus was minimized by reducing the time in the operating room and by moving the baby to the NICU immediately after birth. The NICU and operating room were adjacent, and we could quickly transfer the neonate by keeping her on the arm. However, it is recommended that negative pressure incubator should be used for the transfer [14]. Another reason for the baby’s negative PCR test could be that the transmission of the virus was weakened as the mother’s pre-operative PCR test was turned into a negative result. Furthermore, Li et al. [15] reported that there was no vertical transmission. In our case, the results of SARS-CoV-2 PCR test of placenta, amniotic fluid, and cord blood were all negative.
In a virus pandemic, it is critical to prevent medical staff members from being exposed to viral infections and to provide a safe environment for them. In addition, performing C-sec in pregnant women with significant physiologic changes and managing babies being exposed to pathogen should occur simultaneously. There was no case of C-sec except this case on March 6; hence, we did not prepare for other patients, except for ventilating the delivery room for more than 100 min after surgery.
Therefore, it is essential to establish a formal protocol for managing SARS-CoV-2 infected pregnant women in order to minimize viral exposure and avoid transmission of the virus. It is better to form a multidisciplinary team with designated specialized infection control personnel comprising of surgeon, anesthesiologist, nurse and facility, and equipment technician.
References
1. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382:1708–20.

2. Coronavirus Disease-19, Republic of Korea [Internet]. Osong: Korea Centers for Disease Control and Prevention;[Cited 2020 Mar 27]. Available from http://ncov.mohw.go.kr/.
3. Warning JC, McCracken SA, Morris JM. A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction. 2011; 141:715–24.

4. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020; 395:809–15.

5. Roberge RJ, Kim JH, Powell JB. N95 respirator use during advanced pregnancy. Am J Infect Control. 2014; 42:1097–100.

6. Park J, Yoo SY, Ko JH, Lee SM, Chung YJ, Lee JH, et al. Infection prevention measures for surgical procedures during a Middle East respiratory syndrome outbreak in a tertiary care hospital in South Korea. Sci Rep. 2020; 10:325.

7. Coronavirus [Internet]. Geneva: World Health Organization;[Cited 2020 Mar 27]. Available from http://www.who.int/health-topics/coronavirus.
8. Chen J. Pathogenicity and transmissibility of 2019-nCoV-A: quick overview and comparison with other emerging viruses. Microbes Infect. 2020; 22:69–71.

9. Chow TT, Kwan A, Lin Z, Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect. 2006; 64:371–8.

10. Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005; 54:1–141.
11. Lertakyamanee J, Chinachoti T, Tritrakarn T, Muangkasem J, Somboonnanonda A, Kolatat T. Comparison of general and regional anesthesia for cesarean section: success rate, blood loss and satisfaction from a randomized trial. J Med Assoc Thai. 1999; 82:672–80.
12. Lai HY, Tsai PS, Fan YC, Huang CJ. Anesthetic practice for Caesarean section and factors influencing anesthesiologists’ choice of anesthesia: a population-based study. Acta Anaesthesiol Scand. 2014; 58:843–50.

13. Tompkins BM, Kerchberger JP. Special article: personal protective equipment for care of pandemic influenza patients: a training workshop for the powered air purifying respirator. Anesth Analg. 2010; 111:933–45.
14. Wang L, Shi Y, Xiao T, Fu J, Feng X, Mu D, et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition). Ann Transl Med. 2020; 8:47.

15. Li Y, Zhao R, Zheng S, Chen X, Wang J, Sheng X, et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus-2, China. Emerg Infect Dis. 2020; 26:1335–6.
Fig. 1.
Chest radiograph and computed tomography of the patient. (A) Chest radiograph shows left lower lobe and left middle lobe consolidation and increased vascular marking. (B) Multifocal peribronchial ground glass appearance and consolidation in the left lower lobe is observed on chest computed tomography scans.
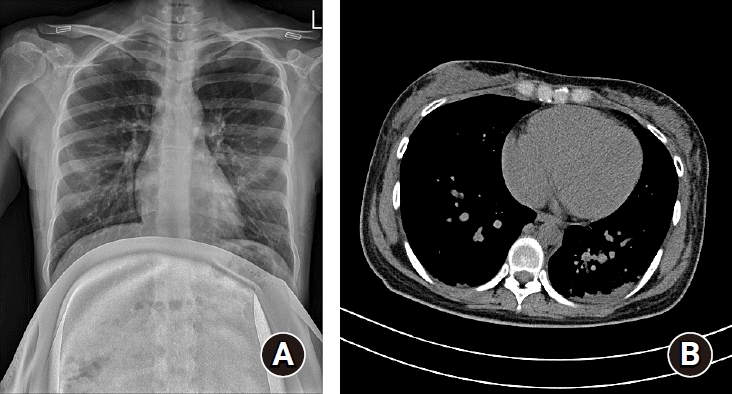
Fig. 2.
Schematic diagram of the delivery center for severe acute respiratory syndrome Coronavirus-2 positive pregnant woman. There are three rooms: (A) pre-treatment room, (B) fitting room for medical staff, and (C) negative pressure operating room. The patient is transferred to a pre-treatment room (A) in the delivery center equipped with negative-pressure ventilation, exclusive elevators, and pre-operative laboratory test kits, including electrocardiogram, blood test, and urine test.
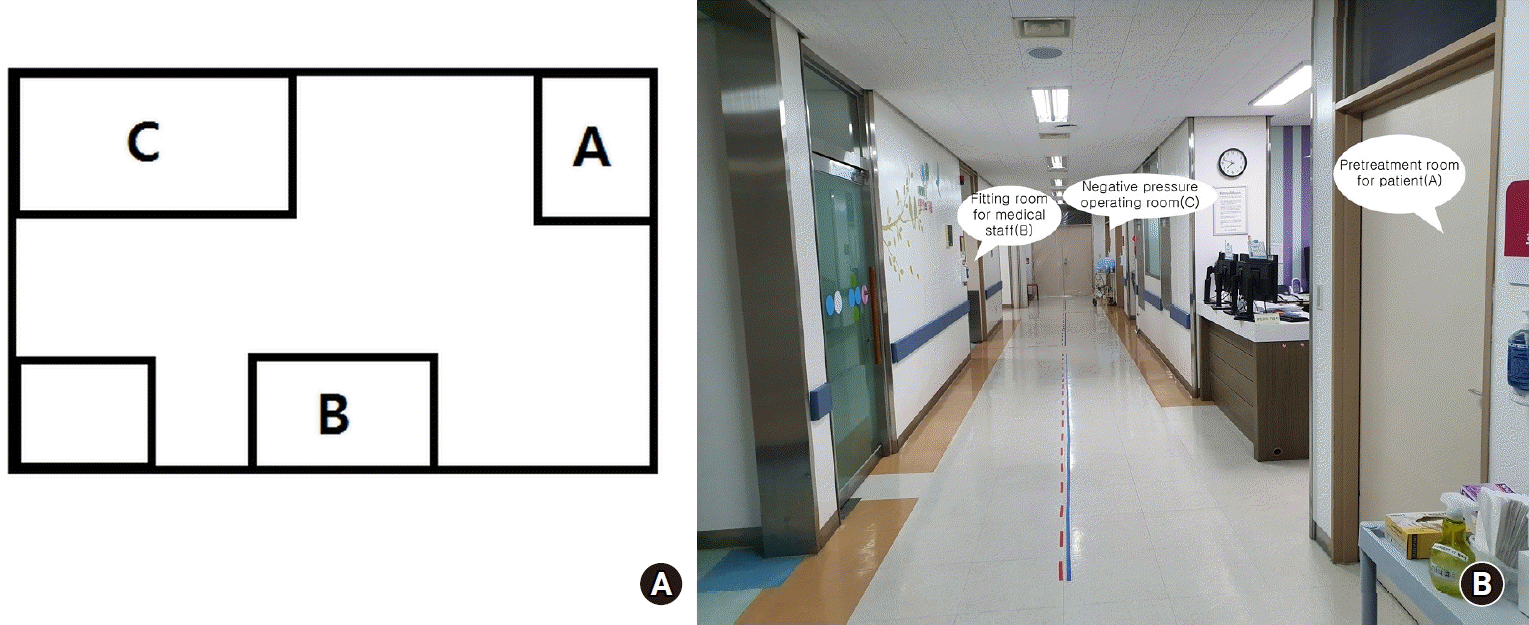
Fig. 3.
Anesthesia with personal protective equipment. (A) All medical staff members wear enhanced personal protective equipment including N95 mask, surgical cap, double gown, double gloves, shoe covers, and a powered air-purifying respirator. (B) Anesthesia is administered by an expert, ensuring that both the anesthesia time and unnecessary virus exposure time is reduced.

Table 1.
Timeline of the Admitted Pregnant Woman with Confirmed Severe Acute Respiratory Syndrome Coronavirus-2




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download