1. Han Y, Smith MT. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front Pharmacol. 2013; 4:156.

2. Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010; 6:657–66.

3. Silva A, Wang Q, Wang M, Ravula SK, Glass JD. Evidence for direct axonal toxicity in vincristine neuropathy. J Peripher Nerv Syst. 2006; 11:211–6.

4. Xu J, Wang W, Zhong XX, Feng Y, Wei X, Liu XG. Methylcobalamin ameliorates neuropathic pain induced by vincristine in rats: effect on loss of peripheral nerve fibers and imbalance of cytokines in the spinal dorsal horn. Mol Pain. 2016; 12:1–14.
5. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998; 50:413–92.
6. Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998; 347:1–11.

7. Lee YW, Yaksh TL. Pharmacology of the spinal adenosine receptor which mediates the antiallodynic action of intrathecal adenosine agonists. J Pharmacol Exp Ther. 1996; 277:1642–8.
8. Pan HL, Xu Z, Leung E, Eisenach JC. Allosteric adenosine modulation to reduce allodynia. Anesthesiology. 2001; 95:416–20.

9. Reshef A, Sperling O, Zoref-Shani E. The adenosine-induced mechanism for the acquisition of ischemic tolerance in primary rat neuronal cultures. Pharmacol Ther. 2000; 87:151–9.

10. Rahn EJ, Makriyannis A, Hohmann AG. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br J Pharmacol. 2007; 152:765–77.

11. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994; 59:369–76.
12. Erichsen HK, Blackburn-Munro G. Pharmacological characterization of the spared nerve injury model of neuropathic pain. Pain. 2002; 98:151–61.
13. Jain V, Jaggi AS, Singh N. Ameliorative potential of rosiglitazone in tibial and sural nerve transection-induced painful neuropathy in rats. Pharmacol Res. 2009; 59:385–92.

14. Thibault K, Elisabeth B, Sophie D, Claude FZ, Bernard R, Bernard C. Antinociceptive and anti-allodynic effects of oral PL37, a complete inhibitor of enkephalin-catabolizing enzymes, in a rat model of peripheral neuropathic pain induced by vincristine. Eur J Pharmacol. 2008; 600:71–7.

15. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994; 53:55–63.

16. Xu JJ, Walla BC, Diaz MF, Fuller GN, Gutstein HB. Intermittent lumbar puncture in rats: a novel method for the experimental study of opioid tolerance. Anesth Analg. 2006; 103:714–20.

17. Park JY, Jun IG. The interaction of gabapentin and N6-(2-phenylisopropyl)-adenosine R-(-)isomer (R-PIA) on mechanical allodynia in rats with a spinal nerve ligation. J Korean Med Sci. 2008; 23:678–84.

18. Ito T, Mochida A, Saito K, Nishi K, Sasaki S, Hisada T, et al. An autopsy case of pulmonary and central nervous system metastatic osteosarcoma treated with thirty-six courses of chemotherapy over four years. Nihon Kokyuki Gakkai Zasshi. 2002; 40:71–6.
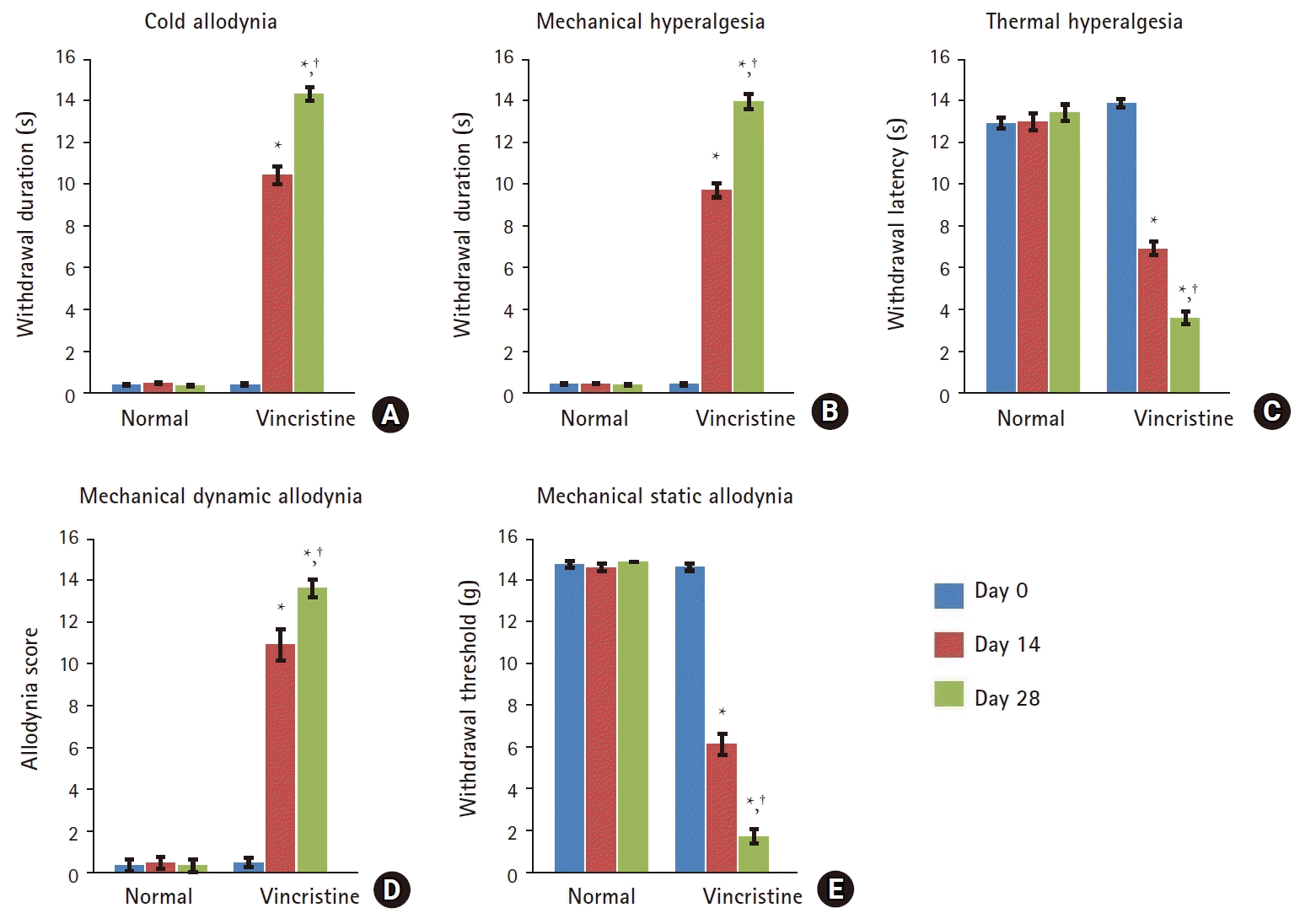
19. Ochoa JL, Yarnitsky D. Mechanical hyperalgesias in neuropathic pain patients: dynamic and static subtypes. Ann Neurol. 1993; 33:465–72.

20. Kaur G, Jaggi AS, Singh N. Exploring the potential effect of Ocimum sanctum in vincristine-induced neuropathic pain in rats. J Brachial Plex Peripher Nerve Inj. 2010; 5:3.

21. Dickenson AH, Suzuki R, Reeve AJ. Adenosine as a potential analgesic target in inflammatory and neuropathic pains. CNS Drugs. 2000; 13:77–85.

22. Zylka MJ. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol Med. 2011; 17:188–96.

23. Lima FO, Souza GR, Verri WA Jr, Parada CA, Ferreira SH, Cunha FQ, et al. Direct blockade of inflammatory hypernociception by peripheral A1 adenosine receptors: involvement of the NO/cGMP/PKG/KATP signaling pathway. Pain. 2010; 151:506–15.

24. Schulte G, Robertson B, Fredholm BB, DeLander GE, Shortland P, Molander C. Distribution of antinociceptive adenosine A1 receptors in the spinal cord dorsal horn, and relationship to primary afferents and neuronal subpopulations. Neuroscience. 2003; 121:907–16.

25. Maione S, de Novellis V, Cappellacci L, Palazzo E, Vita D, Luongo L, et al. The antinociceptive effect of 2-chloro-2’-C-methyl-N6-cyclopentyladenosine (2’-Me-CCPA), a highly selective adenosine A1 receptor agonist, in the rat. Pain. 2007; 131:281–92.
26. Luongo L, Guida F, Imperatore R, Napolitano F, Gatta L, Cristino L, et al. The A1 adenosine receptor as a new player in microglia physiology. Glia. 2014; 62:122–32.

27. Chen JF, Lee CF, Chern Y. Adenosine receptor neurobiology: overview. Int Rev Neurobiol. 2014; 119:1–49.

28. Gessi S, Merighi S, Fazzi D, Stefanelli A, Varani K, Borea PA. Adenosine receptor targeting in health and disease. Expert Opin Investig Drugs. 2011; 20:1591–609.

29. Song JG, Hahm KD, Kim YK, Leem JG, Lee C, Jeong SM, et al. Adenosine triphosphate-sensitive potassium channel blockers attenuate the antiallodynic effect of R-PIA in neuropathic rats. Anesth Analg. 2011; 112:1494–9.

30. Silva IR, Nehlig A, Rosim FE, Vignoli T, Persike DS, Ferrandon A, et al. The A1 receptor agonist R-Pia reduces the imbalance between cerebral glucose metabolism and blood flow during status epilepticus: could this mechanism be involved with neuroprotection? Neurobiol Dis. 2011; 41:169–76.

31. Héron A, Lasbennes F, Seylaz J. Effect of two different routes of administration of R-PIA on glutamate release during ischemia. Neurosci Lett. 1992; 147:205–8.

32. Bai HH, Liu JP, Yang L, Zhao JY, Suo ZW, Yang X, et al. Adenosine A1 receptor potentiated glycinergic transmission in spinal cord dorsal horn of rats after peripheral inflammation. Neuropharmacology. 2017; 126:158–67.

33. Kaur T, Borse V, Sheth S, Sheehan K, Ghosh S, Tupal S, et al. Adenosine A1 receptor protects against cisplatin ototoxicity by suppressing the NOX3/STAT1 inflammatory pathway in the cochlea. J Neurosci. 2016; 36:3962–77.

34. Letson H, Dobson G. Adenosine, lidocaine and Mg2+ (ALM) fluid therapy attenuates systemic inflammation, platelet dysfunction and coagulopathy after non-compressible truncal hemorrhage. PLoS ONE. 2017; 12:e0188144.

35. Viggiano E, Monda M, Viggiano A, Viggiano A, Aurilio C, De Luca B. Persistent facial pain increases superoxide anion production in the spinal trigeminal nucleus. Mol Cell Biochem. 2010; 339:149–54.

36. Seo YJ, Kwon MS, Shim EJ, Park SH, Choi OS, Suh HW. Changes in pain behavior induced by formalin, substance P, glutamate and pro-inflammatory cytokines in immobilization-induced stress mouse model. Brain Res Bull. 2006; 71:279–86.

37. Jaggi AS, Singh N. Role of different brain areas in peripheral nerve injury-induced neuropathic pain. Brain Res. 2011; 1381:187–201.

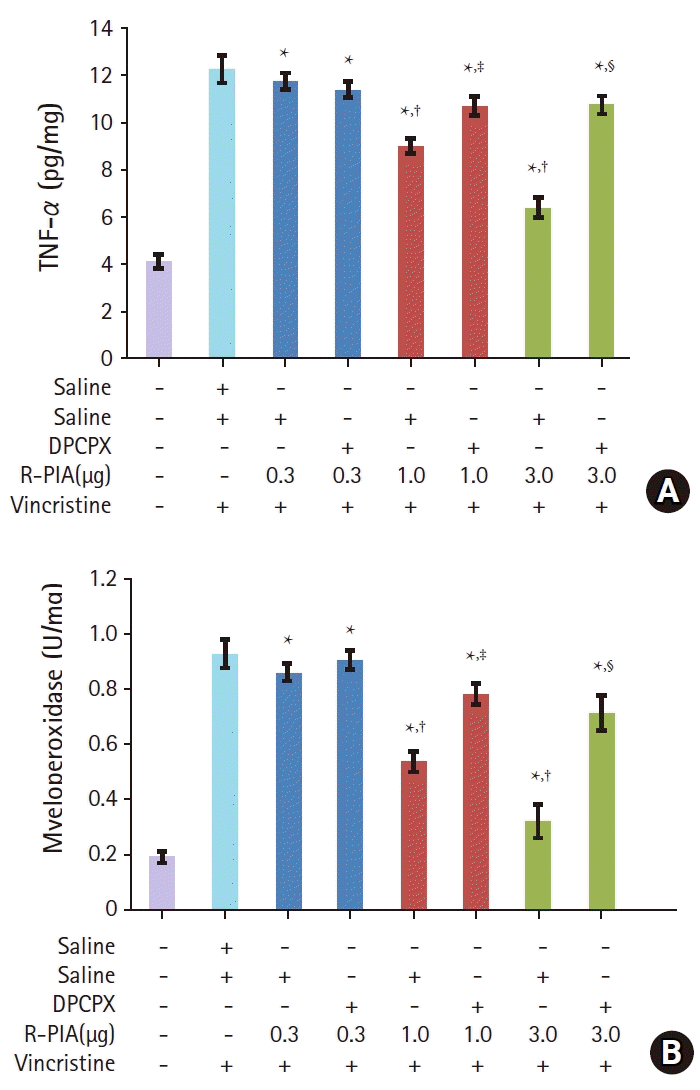
38. Kiguchi N, Maeda T, Kobayashi Y, Kishioka S. Up-regulation of tumor necrosis factor-alpha in spinal cord contributes to vincristine-induced mechanical allodynia in mice. Neurosci Lett. 2008; 445:140–3.

39. Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci. 2002; 22:6696–703.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download