This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The rhomboid intercostal and subserratus plane (RISS) block is a new interfascial block technique that has shown promising results for abdominal and thoracic surgeries. Our objective was to describe the improved analgesia and dermatomal coverage in patients who received bilateral RISS blocks after a major abdominal surgery.
Case
Twenty-one patients who underwent abdominal surgery received the rhomboid intercostal component of the block at the T5 to T6 levels, and the subserratus component block was performed at the T6 to T9 levels. The RISS blocks provided effective postoperative analgesia. There was a variation in the dermatomal coverage ranging from T3 to T12. Patients reported a high satisfaction rate from pain management.
Conclusions
The RISS block in abdominal surgery seems to have an important role in perioperative pain management, complementing the multimodal analgesic regimen. To determine the efficacy of the RISS block for abdominal surgery, we need further randomized control trials.
Go to :

Keywords: Fascial plane block, Interventional ultrasonography, Pain, Pain management, Postoperative pain, Regional anesthesia
Abdominal wall blocks rely on the spread of the local anesthetic within the musculofascial planes to anesthetize multiple small nerves or plexuses, rather than targeting specific nerve structures [
1,
2]. A novel approach for chest wall and upper abdominal analgesia, termed the rhomboid intercostal and subserratus plane (RISS) block, initially showed promising results [
3]. It was reported for different abdominal surgeries, rib fractures, and chest tube-associated pain [
3]. The clinical evaluation of the RISS block demonstrated consistent analgesia from the T5 to T8 dermatome [
3]. In a cadaveric study, the injectate was observed spreading between the intercostal muscles and then deep into the rhomboids and serratus anterior muscles, and staining the lateral cutaneous branches of the intercostal nerves from T4 to T9 [
2]. This emphasizes the role of chest wall blocks as an analgesic modality for the abdominal wall as it is innervated by intercostal nerves [
4]. In this paper, we describe a retrospective case series of patients who received bilateral RISS blocks (either through single-shot injections or through catheter infusion) for analgesia after an abdominal surgery.
Case Report
The Cleveland Clinic Institutional Review Board approved the study (IRB #18-168) and waived the requirement for informed consent. All cases were conducted at the Cleveland Clinic Main Campus during the period between April 2018 and January 2019. The reporting of this study adhered to the Preferred Reporting of Case Series in Surgery Guidelines [
5]. The procedure was conducted on adult patients undergoing an elective major abdominal surgery.
The blocks were performed either preoperatively or postoperatively by one of the investigators (i.e., H.E.). Patients were positioned in the lateral decubitus position and were monitored according to the standard American Society of Anesthesiologists monitoring. The rhomboid intercostal injection was performed using a linear probe ultrasound transducer (6–12 MHz, X-Porte, SonoSite, USA). The transducer was placed in the sagittal plane medial to the medial border of the scapula and then rotated to produce an oblique sagittal view (paramedian sagittal oblique) approximately 1 to 2 cm medial to the medial scapular border. The tissue plane between the rhomboid major and the intercostal muscles was identified. A 17 gauge (G) Tuohy needle was advanced in plane from a superomedial to an inferolateral direction through the trapezius and rhomboid major muscles (
Figs. 1 and
2). Five to 10 ml of the local anesthetic (0.5% ropivacaine or bupivacaine) was administered to the patients receiving a single-injection block on each side, or 5 to 10 ml of 0.2% ropivacaine to the patients receiving a catheter infusion. The rhomboid intercostal component of the block was performed as a single-shot injection at the T5 to T6 levels. The ultrasound probe was then moved caudally and laterally to identify the tissue plane between the serratus anterior and the external intercostal muscle for the subserratus block at the T6 to T9 levels (depending on the desired dermatomal coverage for the lower part of the incision) (
Figs. 3 and
4). The needle was inserted into the same skin entry site as that used for the rhomboid intercostal injection but directed caudally and laterally beyond the inferior angle of the scapula. Fifteen to 20 ml of the local anesthetic was administered (0.5% ropivacaine or bupivacaine for the patients receiving single-shot blocks or 0.2% ropivacaine for the patients receiving a catheter infusion). The target landmark was the plane located superficial to the intercostal muscles. In subjects undergoing a continuous infusion, a 19 G, 40-cm catheter (Arrow
®, Teleflex, USA) was then introduced into the subserratus plane and advanced 3 to 5 cm beyond the needle tip. The catheter tip position was confirmed with an injection of 5 ml of 0.2% ropivacaine under direct ultrasound visualization. The catheters were secured with sterile adhesive dressing. The patient was then repositioned, and the procedure was repeated on the opposite side. During the application of each block, the total local anesthetic dose used per patient was adjusted for the body weight (maximum dose of 3 mg/kg for ropivacaine and 2 mg/kg for bupivacaine).
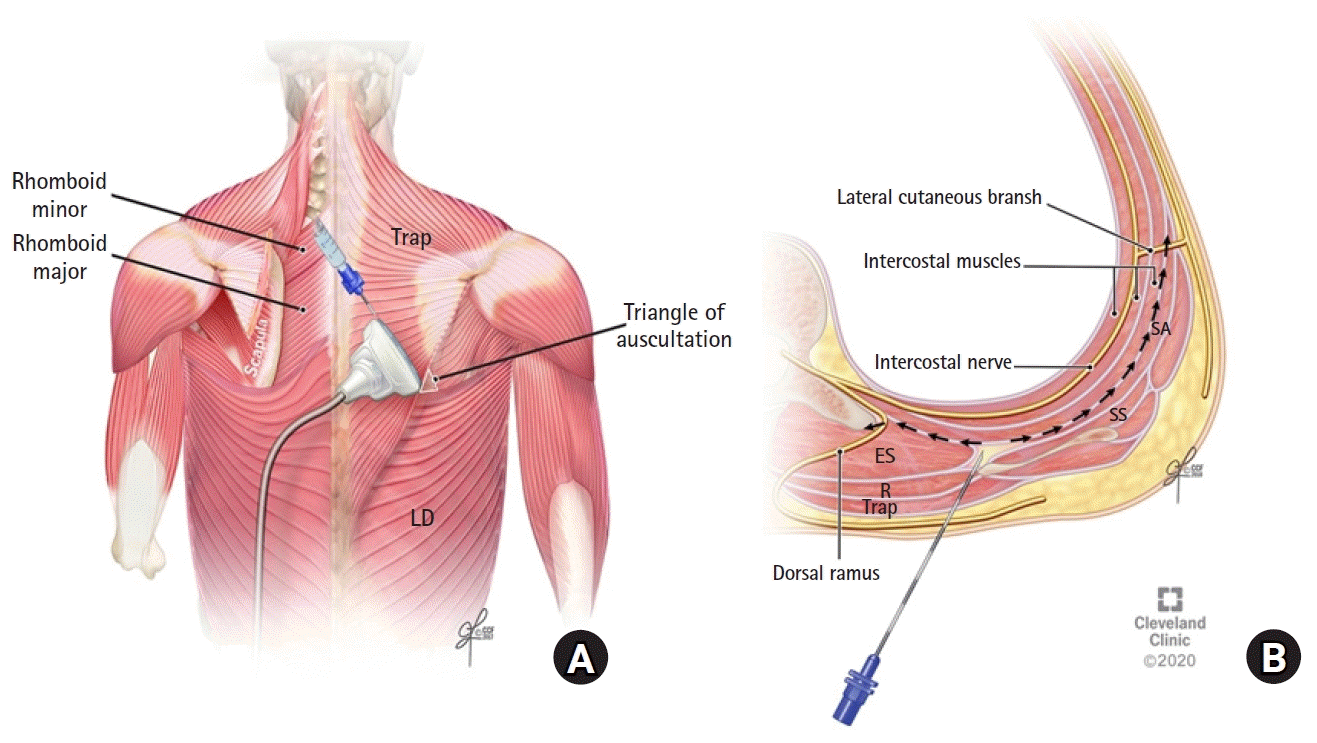 | Fig. 1.(A) Ultrasound transducer position for performing the rhomboid intercostal injection at the T5 and T6 levels. (B) Schematic illustration of an axial section at the T5 to T6 levels showing the needle position and injectate spread during the rhomboid intercostal injection. ES: erector spinae muscle, LD: latissimus dorsi muscle, R: rhomboid muscle, SA: serratus anterior muscle, SS: subscapularis muscle, Trap: trapezius muscle. 
|
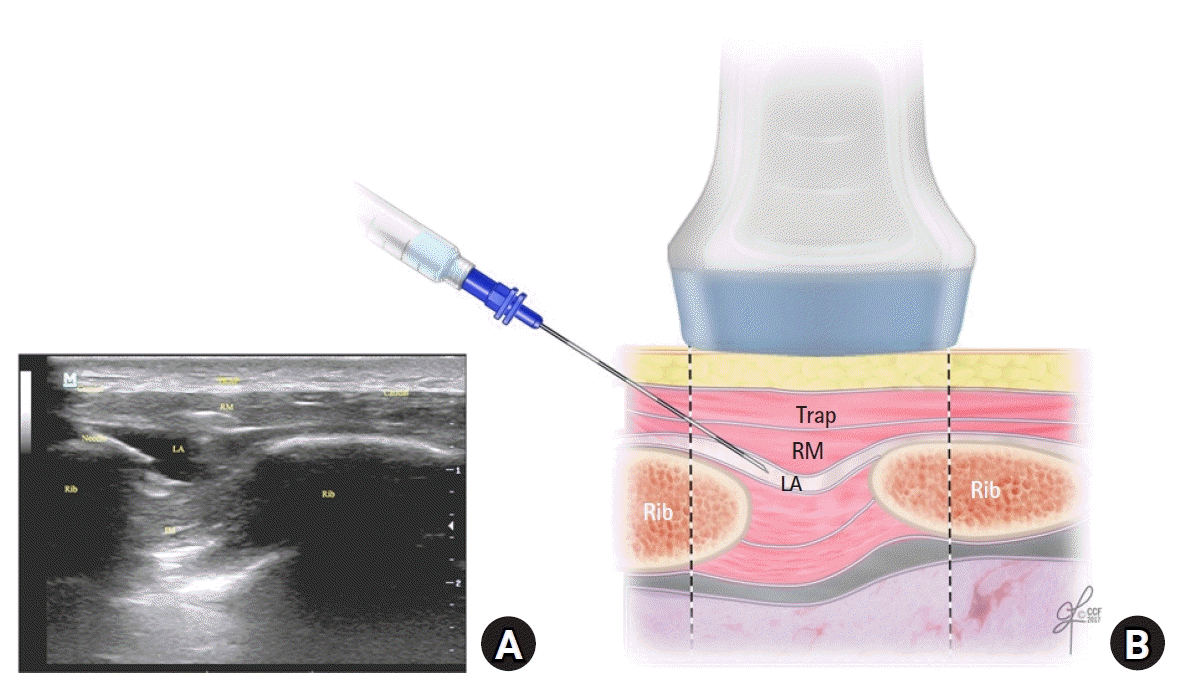 | Fig. 2.(A) The corresponding ultrasound image. (B) Schematic illustration showing the surrounding structures and needle position for performing the rhomboid intercostal injection at the T5 and T6 levels. IM: intercostal muscles, LA: local anesthetic, RM: rhomboid major muscle, Trap: trapezius muscle. 
|
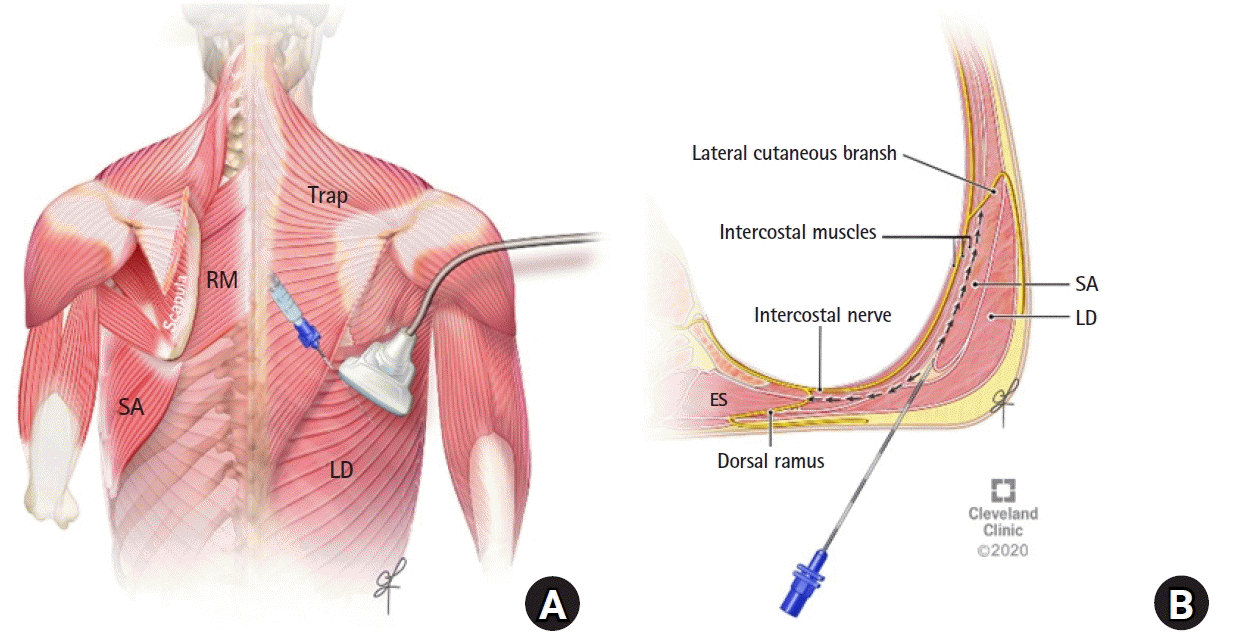 | Fig. 3.(A) Ultrasound transducer position for performing the subserratus plane injection at the T7 and T8 levels. (B) Shematic illustration of an axial section at the T7 to T8 levels showing the surrounding muscle layers and local anesthetic spread. LD: latissimus dorsi muscle, RM: rhomboid major muscle, SA: serratus anterior muscle, Trap: trapezius muscle, ES: erector spinae muscle. 
|
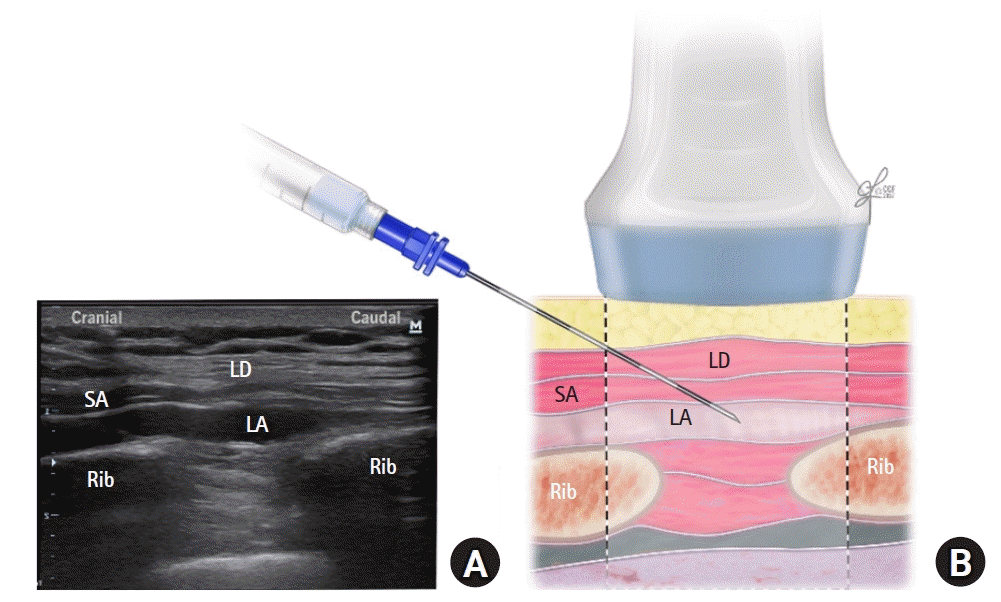 | Fig. 4.(A) Corresponding ultrasound image. (B) Schematic illustration showing the surrounding structures and needle position for performing the subserratus injection at the T7 and T8 levels. LD: latissimus dorsi muscle, SA: serratus anterior muscle, LA: local anesthetic. 
|
For the catheter infusions, each catheter was connected to a patient-controlled infusion pump (Curlin PainSmart™ IOD infusion pump, Curlin Medical, Inc., USA) containing 0.2% ropivacaine at a basal rate of 6 ml/h which was started within 1 h after the operation, with an additional on-demand bolus of 6 ml every 60 min, as needed. Moreover, as per clinical routine, all patients were provided with hydromorphone via an intravenous patient-controlled analgesia (PCA) pump. This PCA pump was started within 1 h from arrival to the post-anesthesia care unit. Pain-at-rest scores using the visual analog scale (VAS) were collected as per clinical routine, with a minimum frequency of once every 4 h. The mean and range of the VAS were reported as a time-weighted average over 24 h for the single-shot blocks and over the local anesthetic infusion duration for the catheter infusion. Outcome data were obtained from the electronic medical records including the overall opioid consumption over 24 h for the single-shot blocks and the RISS catheter infusion. The first evaluation of the patients’ dermatomal coverage was 15 min after the block application on the day of surgery. The extent of the sensory dermatomal block was determined by a diminished cold sensitivity to ice on the side of the trunk compared with that on the ipsilateral arm; it was assessed daily around 9 a.m. by the acute pain team. Additional data regarding the duration of analgesia and possible side effects from the procedure including bleeding, hematoma, hypotension, internal organ injury, catheter site infection, and local anesthetic toxicity were recorded. Data on patients’ satisfaction were obtained from the results of the pain control questionnaire with yes/no answers in the daily report of the acute pain team.
The intervention was administered to 21 opioid-naïve patients (16 males and 5 females; mean age: 59 years [range: 33 to 87 years]). Two patients who were expected to be discharged soon and who underwent less extensive surgery received single-shot blocks, and 19 patients received continuous catheter infusion. Indications were major abdominal surgeries, including exploratory laparotomy and bowel resection (7 patients), pancreaticoduodenectomy (5 patients), hepatectomy (6 patients), gastric bypass (1 patient), ureteral reimplantation (1 patient), and radical nephrectomy (1 patient). A variation in the dermatomal sensory block with a range of coverage from T3 to T12 was achieved. The dermatomal coverage from T7 to T8 was consistent in all the cases (
Table 1). However, we could not evaluate the midline dermatomal coverage owing to the dressing of the midline incision. Data for the mean and range of time-weighted average pain scores, opioid consumption, and duration of the catheter infusion are listed in
Table 1. Most of the patients were satisfied except for 3 patients. There were no reported block-related adverse events or local anesthetic toxicity.
Table 1.
Patient Demographics and Outcomes
|
Patient age (yr)/sex |
Operation |
Range of dermatomal coverage |
Pain scores mean (range) |
Morphine equivalent opioid usage (mg) |
Patients’ satisfaction |
Duration of catheter infusion |
|
1) 33/F |
Gastric bypass |
Right: T4-T10 |
4.7 |
49 |
Satisfied |
6 days |
|
Left: T3-T11 |
(3.9–5.8) |
|
2) 59/F |
Exploratory laparotomy and bowel resection |
Right: T7-T11 |
3.6 |
97.5 |
Satisfied |
4 days |
|
Left: T7-T11 |
(2.8–4.8) |
|
3) 73/M |
Pancreaticoduodenectomy |
Right: T7-T12 |
5.4 |
215.8 |
Not satisfied |
6 days |
|
Left: T7-T12 |
(3.8–7.1) |
|
4) 31/M |
Exploratory laparotomy and bowel resection |
Right: T6-T12 |
3 |
88 |
satisfied |
2 days |
|
Left: T6-T12 |
(2–4.2) |
|
5) 80/M |
Pancreaticoduodenectomy |
Right: T5-T10 |
3.8 |
50 |
Satisfied |
4 days |
|
Left: T5-T10 |
(1.5–6) |
|
6) 65/M |
Pancreaticoduodenectomy |
Right: T6-T9 |
5.3 |
5.3 |
Not satisfied |
Single shot |
|
Left: T6-T9 |
(2.5–6) |
|
7) 63/M |
Pancreaticoduodenectomy |
Right: T6-T10 |
2.5 |
189 |
Satisfied |
4 days |
|
Left: T6-T10 |
(1–3) |
|
8) 47/M |
Pancreaticoduodenectomy |
Right: T7-T10 |
5 |
280 |
Satisfied |
4 days |
|
Left: T7-T10 |
(2–8) |
|
9) 72/M |
Exploratory laparotomy and bowel resection |
Right: T7-T12 |
2.5 |
189 |
Satisfied |
3 days |
|
Left: T7-T12 |
(1–3) |
|
10) 51/M |
Exploratory laparotomy and bowel resection |
Right: T6-T8 |
2.8 |
0 |
Satisfied |
2 days |
|
Left: T6-T8 |
(2.5-3) |
|
11) 83/F |
Hepatectomy |
Right: T7-T10 |
5 |
4 |
Satisfied |
Single shot |
|
Left: T6-T8 |
(1–7) |
|
12) 28/M |
Exploratory laparotomy and bowel resection |
Right: T5-T8 |
6.4 |
88.1 |
Satisfied |
4 days |
|
Left: T5-T9 |
(3.5–9) |
|
13) 40/F |
Hepatectomy |
Right: T6-T12 |
4 |
108.6 |
Satisfied |
3 days |
|
Left: T5-T11 |
(2–6) |
|
14) 64/M |
Exploratory laparotomy and bowel resection |
Right: T5-T9 |
6.5 |
36.7 |
Satisfied |
2 days |
|
Left: T6-T10 |
(6.1–6.9) |
|
15) 77/M |
Hepatectomy |
Right: T7-T10 |
3 |
15.3 |
Satisfied |
4 days |
|
Left: T7-T10 |
(2–4) |
|
16) 57/F |
Ureteral reimplantation |
Right: T7-T11 |
6.4 |
78 |
Satisfied |
3 days |
|
Left: T7-T11 |
(4.8–7.1) |
|
17) 47/M |
Exploratory laparotomy and bowel resection |
Right: T6-T10 |
6.7 |
770.3 |
Not satisfied |
2 days |
|
Left: T8-T10 |
(6.3–7.2) |
|
18) 87/M |
Hepatectomy |
Right: T6-T9 |
0.8 |
7.5 |
Satisfied |
5 days |
|
Left: T6-T9 |
(0–1.2) |
|
19) 41/M |
Hepatectomy |
Right: T3-T8 |
5.25 |
65 |
Satisfied |
1 day |
|
Left: T4-T7 |
(5.2–5.3) |
|
20) 62/M |
Pancreaticoduodenectomy |
Right: T5-T9 |
6 |
16.3 |
satisfied |
1 day |
|
Left: T5-T9 |
(5–7) |
|
21) 40/M |
Radical nephrectomy |
Right: T6-T10 |
4.1 |
114 |
Satisfied |
2 days |
|
Left: T6-T10 |
(3.5–5) |

Go to :

Discussion
In this paper, we report a retrospective case series of adult patients having a major abdominal surgery provided with bilateral RISS blocks for postoperative analgesia. The intervention was found to obtain a reproducible dermatomal sensory analgesic coverage from T7 to T8 bilaterally in all the 21 cases, irrespective of the level of needle application, which could be anywhere from approximately T5 to T6 for the rhomboid intercostal block and from T6 to T9 for the subserrate block. Moreover, we recorded a variation in the dermatomal coverage, which ranged from T3 to T12. The procedure was well tolerated by the patients, most of whom reported a high satisfaction rate from the pain management except for three patients. Because our case series consisted of a heterogeneous group of patients who underwent different types of abdominal surgery, it may serve as a pioneering example for other randomized clinical trials.
Since the original publication of the RISS block [
6], several studies on patients undergoing different operations such as lung transplantation [
7], back surgery [
8], thoracotomy [
9], transapical transcatheter aortic valve implantation [
10], breast reconstruction surgery [
11], and modified radical mastectomy [
12] with axillary curettage have shown that the RISS block may generate analgesia from the T2 to T9 dermatomes.
A previous cadaveric study provided an evidence that the tissue plane deep into the erector spinae muscle, rhomboid muscles, serratus anterior muscles, latissimus dorsi, and upper part of the external oblique muscle is continuous [
3]. In the RISS block, the injectate is directed toward the tissue plane located between the rhomboid and the intercostal muscles, and then deep into the scapula and the serratus anterior muscle, which targets the lateral cutaneous branches of the ventral rami of the thoracic intercostal nerves [
3]. The spread extends medially deep into the erector spinae tissue plane and superficial to the thoracic transverse processes at the point where the dorsal rami of the thoracic intercostal nerves emerge between the tips of the adjacent transverse processes from T3 to T9. Moreover, there are many factors that limit the cadaveric studies from exploring the extent of the dye injected. This is mainly because of the absence of the biomechanical properties in the cadaver tissues [
2]. However, theoretically, as the local anesthetic spreads medially deep into the erector spinae muscle, it can penetrate deep into the paravertebral space and block other nerves including the anterior and motor branches of the ventral rami of the thoracic intercostal nerves.
Previous chest wall blocks such as the intercostal nerve [
13] and thoracic paravertebral [
14] blocks have been described for analgesia after upper abdominal surgeries. The RISS block offers an advantage over the transverse abdominus plane (TAP) block as the latter does not provide consistent coverage to the lower thoracic dermatome areas. Even the subcostal TAP does not cover the lateral supraumbilical area [
15]. Moreover, the clinical advantage of the RISS block is that the point of injection is far from most surgical incisions, and a catheter is unlikely to interfere with the surgical field [
3]. Moreover, compared with the erector spinae plane block, the RISS block is technically easier to administer in terms of patient positioning, defining the landmark (borders of the scapula), and spread of the local anesthetics between the interfacial planes of the rhomboid major or serrates anterior from one side and the external intercostal muscle from the other side. Moreover, it is less invasive than the epidural block and paravertebral blocks, which makes the RISS block theoretically less susceptible to hemodynamic instability, bleeding, local anesthetic systemic toxicity, or permanent nerve damage.
We recognize some limitations to the case series, the main one being that it is a retrospective review. Moreover, the different incisions and surgical procedures might have affected the variability of the results. Lastly, all the blocks were done by one investigator (i.e., H.E.).
The RISS technique was shown to be beneficial for patients in this case series; further randomized clinical trials are needed, particularly a comparison with neuraxial and interfascial plane blocks.
Go to :








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download