Abstract
Urologic surgeries are widely performed, and the cases have increased owing to the fact that the elderly population is growing. The narrow and limited surgical space is a challenge in performing most urologic surgeries. Additionally, the elderly population is exposed to the risk of perioperative complications; therefore, a comprehensive understanding and approach are required to provide optimized anesthesia during surgery. We have searched the literature on anesthesia for urologic surgeries and summarized the anesthetic considerations for urologic surgeries.
Go to : 
Most urologic surgeries are performed in a narrow and limited space with the minimally invasive technique or cystoscope, and most patients undergoing urologic surgeries are elderly individuals with other diseases. Therefore, anesthesiologists should, as well as provide adequate anesthesia, consider various factors such as age, co-morbidities, functional status, duration of surgery, predicted blood loss and surgical scope, to optimize surgical outcomes. This review aimed to provide the anesthetic considerations for various urologic surgeries.
Go to : 
From 1999 to 2012, in Korea, the overall incidence rates of kidney, bladder, and prostate cancer were 4.4%, 4.79%, and 7.75% [1] and generally increase with aging [2]. Owing to a growing elderly population, there has been an increase in the number of urologic surgeries, such as nephrectomy, cystectomy, and prostatectomy [3]. Surgery has been considered to be associated with higher risk of complications in elderly patients. Moreover, elderly patients tend to have comorbidities. Since these high-risk patients account for 80% of postoperative deaths [4], perioperative care, such as risk stratification, adequate intraoperative intervention, and prevention of postoperative complications, plays an important role in improving surgical outcomes, morbidity, and mortality [5].
The essential step is to identify patients who have a high risk of complications. Preoperative assessment of functional status is important because impaired physical function increases the risk of postoperative complications [6], delirium [7], and surgical site infection [8]. Patients with poor physical status should be further evaluated and pretreated to enhance functional capacity for optimized recovery [9].
Frailty reflects decreased physiologic reserve and increased vulnerability to poor health outcomes [10]. Several studies reported that frailty was significantly associated with postoperative mortality and morbidity in patients undergoing urologic surgery [11-13]. Therefore, preoperative frailty assessment may be useful in predicting postoperative outcomes.
Several factors associated with postoperative complications can be modified preoperatively. Smoking cessation before surgery reduces respiratory complications and promotes wound healing [14]. A smoker should be advised to stop smoking at least 4 weeks before surgery [15]. Patients scheduled for urologic surgery may have iron deficiency, with or without anemia [3]. Preoperative iron deficiency and anemia could increase the incidence of blood transfusion [3], which is reported to be associated with postoperative mortality and morbidity [16]. Therefore, treatment of iron deficiency, with or without anemia, is recommended in case of predictive blood loss > 500 ml [17]. Preoperative oral iron supplements may reduce perioperative transfusion and improve surgical outcomes.
Go to : 
Nephrectomy is a standard treatment for renal cell carcinoma (RCC). Partial or radical nephrectomy may be performed according to the tumor characteristics. The European Association of Urology guidelines recommended that patients with mass < 4 cm may undergo partial nephrectomy [18]. Since RCC commonly presents in patients aged > 70 years, most patients undergoing nephrectomy are elderly with comorbidities [19]. An evaluation of other medical conditions, such as cardiovascular, pulmonary, and cerebrovascular diseases, is required preoperatively. It could be important in estimating residual renal function because the whole or part of the kidney will be removed.
Patients are commonly placed in lateral decubitus position during nephrectomy and exposed to pressure sores, nerve damage, or venous congestion, which should be prevented with caution. For example, the eyes and ears should be protected from excessive pressure, brachial plexus injury should be prevented by applying axillary roll on the dependent side, and the neck should be placed in neutral position [20].
Since robotic surgery had been introduced into the surgical field, robot-assisted nephrectomy has gained popularity and has been increasingly performed over the decades [21]. Considerable space must be guaranteed because the robot system is heavy and bulky. The range of robot arms can be wide, so the patient’s head should be protected from unexpected collisions with the robot arms. Robot docking may disturb patient assessment and immediate management, particularly in an emergency situation. Patient movement may lead to tissue injury during robot docking [22]. Thus, sufficient neuromuscular blockade (NMB) should be considered to prevent movement or muscle contraction.
Administration of nonsteroidal anti-inflammatory drugs (NSAIDs) is commonly avoided in patients with kidney surgery due to their nephrotoxic effects. However, NSAIDs have not only postoperative analgesic effect but also opioid-sparing effect, which could decrease side effects associated with opioid use [23]. A previous study reported the favorable effects of NSAIDs in patients undergoing nephrectomy [24]. Freeland et al. [24] analyzed patients undergoing live-donor nephrectomy and found that those who received ketorolac postoperatively had less postoperative pain and shorter length of hospital stay (LOS) without reduction in renal function.
Go to : 
Cystectomy is the treatment of choice for invasive bladder cancer. According to the surgical type, the whole or part of the bladder may be removed. It is a long and complicated procedure with bleeding risk. The average blood loss during cystectomy is from 0.56 to 3 L [25, 26]. Therefore, blood transfusion might be considered, if necessary. However, blood transfusion was significantly associated with lower 5-year recurrence-free survival, cancer-specific survival, and overall survival in a previous study that analyzed 2,060 patients who underwent radical cystectomy [27]. Another study that included 2,934 patients who underwent radical cystectomy also revealed that perioperative blood transfusion increased morbidity and surgical site infection [28]. In contrast, Abel et al. [29] evaluated the association between the timing of blood transfusion and outcomes, and the results showed that intraoperative blood transfusion significantly increased the risk of cancer recurrence and mortality, whereas postoperative transfusion did not. In contrast to previous studies, several studies insisted the insignificant association between blood transfusion and cancer-related outcomes [30, 31]. Although blood transfusion increased recurrence and mortality in the univariate analysis, this association no longer remained significant in the multivariate or adjusted analysis.
Patients with ileal conduit urinary diversion are susceptible to acid-base disorders. Hydrogen ion, chloride ion, or ammonia in the urine could be reabsorbed from the ileal conduit, which would induce hyperchloremic metabolic acidosis [32]. Van der Aa et al. [32] reported that alkalizing agents blocking chloride transport could be used to treat acid-base disorders.
Go to : 
Transurethral resection of bladder cancer (TURB), an endoscopic procedure, is the cornerstone in the diagnosis and treatment of bladder cancer [33]. TURB is performed in a narrow and limited bladder space, and the shape, size, location, and number of tumors can be identified though the procedure. The obturator nerve running close to the lateral wall of the bladder may be stimulated during TURB, which may result in obturator nerve reflex and unpredictable movement of the ipsilateral thigh. Therefore, appropriate anesthesia should be provided for adequate surgical condition and complete resection during TURB. TURB may be performed under either general or regional anesthesia.
General anesthesia with propofol and desflurane offers more rapid induction and recovery in elderly patients undergoing brief transurethral surgery compared to spinal anesthesia [34]. NMB is needed for endotracheal intubation or supraglottic airway device. Additionally, adequate depth of NMB is required to prevent obturator nerve reflex, which causes unpredictable adductor muscle contraction. Cesur et al. [35] reviewed and analyzed 89 patients who underwent TURB from 1997 to 2007. Among them, 56 patients underwent TURB under general anesthesia and were all administered succinylcholine (depolarizing NMB agent) before resection. The authors reported that complete resection was performed in all patients. Koo et al. [36] conducted a randomized controlled trial on rocuronium (nondepolarizing NMB agent), comparing the surgical conditions and incidence of obturator nerve reflex according to the depth of NMB during TURB under general anesthesia. The authors demonstrated that deep NMB significantly increased optimal surgical condition and decreased the incidence of obturator nerve reflex compared to moderate NMB. The bladder consists of smooth muscles where NMB agents are ineffective. Therefore, it is inferred that full relaxation of surrounding muscles, including the pelvis and abdomen, could enhance surgical conditions.
Many transurethral surgeries were successfully performed under spinal anesthesia. In a previous study on patients who underwent urologic surgery, spinal anesthesia with hyperbaric bupivacaine 12 mg with 3 µg of dexmedetomidine or 30 µg of clonidine provided effective anesthetic effect with preserved hemodynamic stability [37]. Other studies showed that spinal anesthesia using levobupivacaine also offered sufficient anesthetic effect during transurethral surgery [38,39]. However, spinal anesthesia could not prevent obturator nerve reflex, and obturator nerve block (ONB) is required to prevent obturator nerve reflex. One previous study compared the incidence of obturator nerve reflex between spinal anesthesia and spinal anesthesia combined with ONB and showed that the incidence of obturator nerve reflex was lower in the patient who received spinal anesthesia combined with ONB (40% vs. 11.4%) [40].
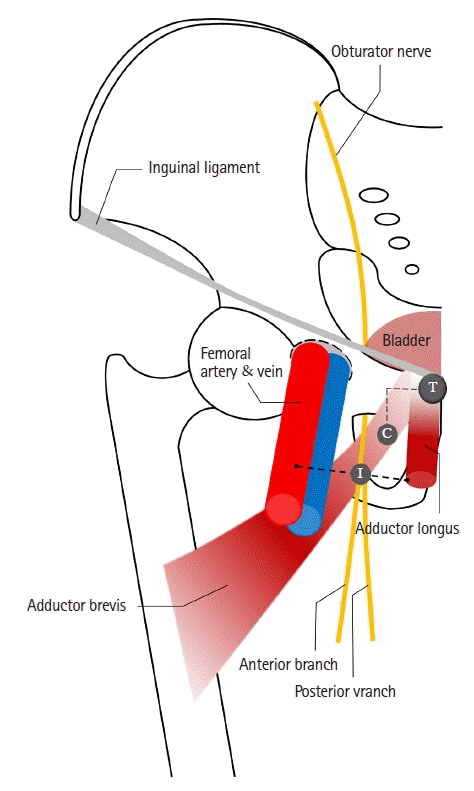
There are various techniques for ONB, depending on the insertion point and needle direction. Fig. 1 shows the classic and inguinal approaches in ONB . Labet introduced a pubic approach, which is known as the classic approach [41]. The needle is inserted at 3 cm lateral and 3 cm inferior to the pubic tubercle and advanced to the ramus of the pubis [42]. The obturator nerve was blocked at the obturator foramen [43]. In especially obese patients or patients with blunt tubercle, it may be difficult to identify the pubic ramus, the landmark of the classic approach. The classic approach may result in injury to adjacent organs, such as the bladder, rectum, and spermatic cord [44]. The inguinal approach was first described by Choquet et al. [45] in 2005, and the needle is inserted at the midpoint of the line between the ipsilateral femoral arterial pulse and inner border of the adductor longus tendon. The obturator nerve is blocked between the adductor brevis and adductor magnus [43]. In previous studies comparing the two methods, the inguinal approach was associated with a higher success rate and lower number of attempts than the classic approach [44,46]. Other methods, such as inter-adductor approach (needle is inserted at the upper end of the adductor longus) and intravesical approach (obturator nerve is blocked through the cystoscope), have also been studied [47,48].
Go to : 
Transurethral resection of the prostate (TURP) is the gold standard treatment for benign prostatic hyperplasia. Like TURB, it can be performed under either general anesthesia or spinal anesthesia. Since TURP is performed in narrow and limited spaces, irrigating fluid is used for bladder distension and sufficient surgical view. However, adverse events related to the use of irrigating fluids may occur in patients undergoing TURP [49]. Venous sinus exposure and prostatic capsule injury allow the absorption of irrigating fluid in the body. The absorbed irrigating fluid can cause acute change in the intravascular volume, electrolyte concentration, and osmolality, which leads to complications such as fluid overload, pulmonary edema, hyponatremia, and coagulopathy. Additionally, the additives of irrigating fluid, such as glycine and sorbitol, are metabolized to ammonia, which may induce tremor or seizure. This phenomenon is called TURP syndrome. The incidence rate of TURP syndrome is reported to be 1–8% [50]. TURP syndrome may cause several symptoms, including headache, anxiety, vomiting, dyspnea, arrhythmia, hypotension, confusion, seizure, and coma [51]. If any of the abovementioned symptoms occur, the anesthesiologist should suspect the development of TURP syndrome and discontinue the surgery and fluid administration. However, if patients are under general anesthesia during TURP, it is difficult to observe the symptoms of TURP syndrome. Laboratory tests may be considered to check serum sodium concentration or serum osmolality. Shin et al. [52] found that rotational thromboelastometry was useful in detecting coagulopathy caused by TURP syndrome. The treatment of TURP syndrome is supportive care, including respiratory support and anticonvulsant and adrenergic drug use. According to the severity of TURP syndrome, diuretics or hypertonic saline could be administered [51].
Go to : 
Since robotic surgery had been introduced to the surgical field in 1999 [53], robot-assisted laparoscopic radical prostatectomy (RALP) has been widely performed to treat prostatic cancer [54]. Most prostatectomies are currently performed by robot-assisted surgery [55]. For optimal surgical view, RALP requires 30° Trendelenburg position and high-pressure pneumoperitoneum, which moves abdominal organs to cephalad. Chest banding prevents falls. Anesthesiologists should understand the physiologic alterations caused by position, pneumoperitoneum, and chest banding. Function residual capacity and lung compliance decrease and induce ventilation-perfusion mismatch, atelectasis, and hypercapnia [56]. Several studies suggested appropriate ventilator strategies that improve oxygenation and reduce CO2 [57]. Jo and Kwak [58] recommended pressure-controlled ventilation rather than volume-controlled ventilation to improve respiratory mechanics or oxygenation during pneumoperitoneum. Ahn et al. [59] demonstrated that the recruitment maneuver could improve intraoperative oxygenation in patients undergoing RALP. Kim et al. [60] revealed that prolonged inspiratory phase, for example, 2 : 1 and 1 : 1, could provide better oxygenation and better CO2 elimination during pneumoperitoneum. Lee et al. [61] found that 7 cmH2O of positive end-expiratory pressure increased oxygenation without excessive peak airway pressure. Moreover, peak airway pressure should be maintained at < 35 cmH2O [62].
The Trendelenburg position may increase venous return and central venous pressure, which may increase cardiac output. Conversely, pneumoperitoneum may reduce cardiac output by increased systemic venous resistance. Falabella et al. [63] found slight but not significant reduction in cardiac output with the Trendelenburg position with pneumoperitoneum in patients undergoing RALP.
The Trendelenburg position could increase intracranial pressure, so a careful approach is required in patients who have a history of aneurysm or stroke. Mavrocotados et al. [64] reported that the intracranial pressure increased from 8.8 mmHg to 13.3 mmHg after a 30° head-down position. Intraocular pressure can also increase; thereby, corneal abrasion or optic neuropathy could develop [65–67]. Additionally, anesthesiologists should also consider any potential risks of the development of subcutaneous emphysema, pneumothorax, or pneumomediastinum.
During RALP, the bladder is opened to access the prostate. Since excessive urine may disturb the surgical procedure, fluid administration is restricted for optimal surgical view. Gainsburg et al. [68] insisted that < 800 ml of fluid should be administered until anastomosis of the bladder and urethra.
Go to : 
Percutaneous nephrolithotomy (PCNL) is commonly performed to treat renal stone. The indication of PCNL includes > 1.5–2 cm of renal calculi, staghorn calculi, lower pole stone, and refractory upper tract calculi [69, 70]. Patients are placed in prone position under either general or spinal anesthesia. The advantages of general anesthesia are securing airway even in prone position and minimizing pleural injury by control of tidal volume during the procedure [70]. However, there is a risk of pressure on the eyes, ears, nose, and any bony structure in the prone position. Thus, abovementioned areas should be protected throughout the surgery. In contrast to general anesthesia, spinal anesthesia may provide better analgesia and shorter recovery time [70]. The eyes, ears, and nose can be protected by the patient because they are awake during surgery. However, patients can complain of discomfort in case of prolonged surgery or insufficient anesthesia. Spinal anesthesia may aggravate unstable hemodynamics in patients with comorbidities. A recent meta-analysis revealed that the regional anesthesia group showed shorter operative time, lower postoperative pain, lesser analgesic requirements, and shorter LOS compared to the general anesthesia group [71]. However, the incidence of hypotension was significantly higher in the regional anesthesia group than that in the general anesthesia group. The stone-free and total complication rates were comparable between the two groups. The complications of PCNL include pleural injury, whose incidence rate is up to 3.1%, small bowel injury, colon injury, hepatic injury, or splenic injury [70]. Theses complications may lead to sepsis or peritonitis, unless early detection and immediate intervention are performed. During the procedure, bleeding may originate from the renal capsule or parenchyma. A potential risk for major bleeding is associated with scanty parenchyma or proximity of major vessels [69]. Srivastava et al. [72] reviewed 1854 patients undergoing PCNL and reported that 1.4% of patients underwent angioembolization due to major bleeding. Therefore, adequate hydration may be useful in maintaining stable hemodynamics.
Ureteroscopy (URS) is used to diagnose and treat problems of the urinary tract, such as ureteral stones. URS distends the renal capsule, ureter, and renal collecting system; stimulates the nociceptors; and produces pain and reflex muscle spasm. This results in flank, groin, scrotal, or labial pain. Therefore, URS should be performed under adequate anesthesia. There are several studies on URS successfully performed under local anesthesia combined with intravenous sedation [73–75]. However, several factors (short duration, small caliber, female sex, experience of the urologist, etc.) are associated with successful completion of URS [76]. General anesthesia prohibits patient movement and breathing, thereby decreasing the risk of urethral trauma. Spinal anesthesia is not preferred in patients undergoing URS because of increased induction time and delayed recovery time [76].
Go to : 
Urologic surgeries commonly produce mild to moderate pain [22]. Pain control is one of the important factors affecting the quality of recovery. There are many studies investigating various methods to alleviate pain in patients undergoing urologic surgeries [77]. In previous studies, transversus abdominis plane (TAP) block provided good analgesic effect and reduced opioid consumption in patients undergoing minimally invasive surgery [78, 79]. Local anesthetics are delivered into the layer between the internal oblique and transversus abdominis. They block sensory pathways of intercostal nerves T7–T11, subcostal nerve T12, and ilioinguinal and iliohypogastric nerves L1, which are innervated to the anterolateral abdominal wall. Baeriswyl et al. [80] conducted a meta-analysis to compare the analgesic efficacy of TAP block and epidural analgesia. There was no significant difference in pain score at postoperative day 1 between the two groups, whereas the incidence rate of hypotension was significantly lower and LOS was shorter in the TAP block group compared to those in the epidural analgesia group. In another study, TAP block significantly reduced pain at postoperative day 1 and opioid consumption and shortened LOS in patients undergoing RALP [81,82]. TAP block was also proved to decrease the first 24-h mean pain score after minimally invasive nephrectomy [79, 83-85]. Matulewicz et al. [86] reported that enhanced recovery after surgery (ERAS) protocol with TAP block improved bowel movement and decreased opioid consumption.
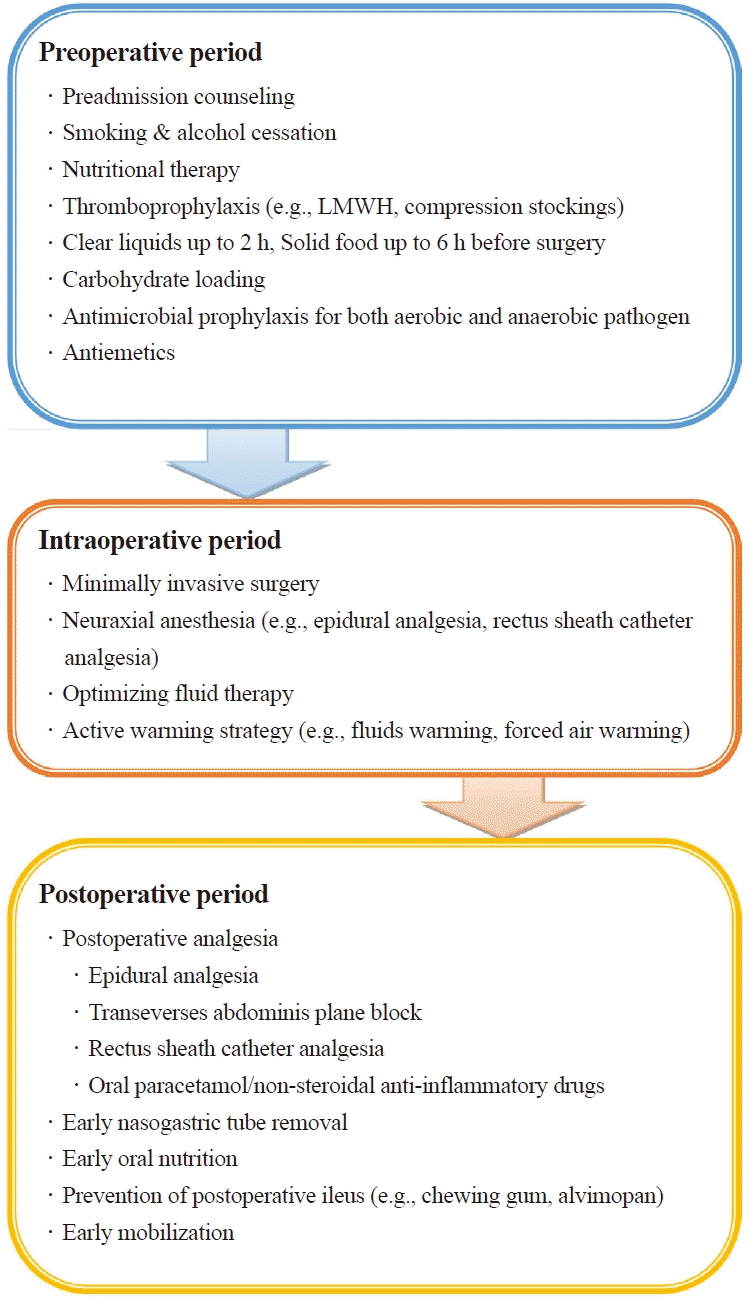
Since ERAS protocol had been introduced to patients undergoing colorectal surgery [87], this new approach has been applied to other types of surgeries. It is a new multimodal approach to improve preoperative status and perioperative homeostasis [88]. Patients undergoing urologic surgeries required optimized ERAS protocol because of several reasons [88]. In terms of surgical factors, urologic surgeries have longer operative time, increased risk of bleeding, and higher complication rates. Regarding patient factors, patients undergoing urologic surgeries are usually elderly with comorbidities, anemia, or malnutrition. Fig. 2 presents the ERAS protocol in patients undergoing urologic surgeries. There are still issues in reaching a consensus on the ERAS protocol in urologic surgeries, and further investigation is needed.
A urinary catheter is often placed for postoperative drainage after surgery. However, a urinary catheter usually irritates the bladder and induces patient discomfort, which is known as catheter-related bladder discomfort (CRBD) with reported incidence rate from 47% to 90% [89]. CRBD may lead to emergence agitation [90]; therefore, preventing CRBD may contribute to better quality of recovery. Several pharmacologic interventions have preventive effects on CRBD. Nefopam, a non-opioid analgesic, inhibits reuptake of dopamine, norepinephrine, and serotonin, and 20 mg of nefopam administered before spinal anesthesia decreased the incidence and severity of CRBD [91]. Cheon et al. [92] used 40 mg of nefopam to prevent CRBD and found that intravenous nefopam could reduce not only the incidence and severity of CRBD but also postoperative pain and rescue drug requirements. Parecoxib, a cyclooxygenase-2 selective inhibitor, has been used to alleviate postoperative pain, and intravenous administration of 40 mg parecoxib reduced the incidence and severity of CRBD postoperatively [93]. Another study demonstrated that continuous infusion of dexmedetomidine reduced the incidence of CRBD [94]. Gabapentin, a structural analogue of gamma-aminobutyric acid, inhibits peripheral sensitization of afferent C-fiber, which is associated with overactive bladder, urge incontinence, and sensory urgency [95, 96], and 600 mg gabapentin decreased the incidence of CRBD from 90% to 66%, while 1,200 mg gabapentin decreased the incidence from 90% to 26% [97]. Patients premedicated with glycopyrrolate also showed decreased postoperative pain, incidence, and severity of CRBD [98].
During transurethral procedures, such as TURB, TURP, or URS, intraoperative penile erection may delay the procedures and lead to complications, such as bleeding and stricture formation, although it rarely occurs (0.1–2.4%) [99]. Various strategies for intraoperative erection have been suggested. Ethyl chloride or dorsal nerve block was described as a method to reduce sensory input to the penis [100, 101]. Intracavernous injection (phenylephrine, epinephrine, norepinephrine) or intravenous injection (ephedrine, dexmedetomidine, glycopyrrolate, ketamine) are described as pharmacological treatments [99]. Close hemodynamic monitoring is needed during intracavernous or intravenous injection of the drug.
Go to : 
Transurethral procedures can be performed under either general or spinal anesthesia, and several studies have shown that the prognosis depends on the type of anesthesia. Jang et al. [102] compared the prognosis of bladder cancer in general and spinal anesthesia in patients undergoing TURB and concluded that spinal anesthesia provided a higher 5-year survival rate than general anesthesia. Other studies also suggested that spinal anesthesia was associated with decreased recurrence rate and extended recurrence-free survival compared to general anesthesia [103, 104]. These results may be because inhaled anesthetics during general anesthesia may suppress immunity, impair host defense, and proliferate malignant cells [105]. Inhaled anesthetics have been known to inhibit natural killer cell activity, monocyte phagocytosis, and tumoricidal activity [106-108], whereas they release hypoxic inducible factor-1 [105]. Hypoxic inducible factor-1 stimulates protumorigenic behavior in residual cancer cells and contributes to recurrence [105]. Another explanation of the advantage of spinal anesthesia may be attributed to the anti-metastatic effect of local anesthetics, such as lidocaine and ropivacaine, which was demonstrated in the in vitro study by Piegeler et al. [109]. However, since most transurethral procedures are relatively short, it is difficult to determine the effect of anesthesia duration on cancer recurrence and survival rate. Therefore, long-term, large-scale, and prospective investigations are needed to establish the effect of anesthesia on recurrence and survival rates.
Go to : 
Urologic surgeries include various spectrums of disease and elderly patients. Therefore, overall collaboration between the urologist and the anesthesiologist is required in terms of preoperative evaluation, intraoperative management, and postoperative care. An individualized, optimized approach leads to better outcomes, quality of recovery, and patient satisfaction.
Go to : 
Notes
Author Contributions
Chang-Hoon Koo (Conceptualization; Investigation; Methodology; Writing – original draft)
Jung-Hee Ryu (Conceptualization; Investigation; Methodology; Supervision; Writing – review & editing)
Go to : 
References
1. Joung JY, Lim J, Oh CM, Jung KW, Cho H, Kim SH, et al. Current trends in the incidence and survival rate of urological cancers in Korea. Cancer Res Treat. 2017; 49:607–15.

2. Brodak M, Tomasek J, Pacovsky J, Holub L, Husek P. Urological surgery in elderly patients: results and complications. Clin Interv Aging. 2015; 10:379–84.
3. Cui HW, Turney BW, Griffiths J. The preoperative assessment and optimization of patients undergoing major urological surgery. Curr Urol Rep. 2017; 18:54.

4. Findley GP. Knowing the risk: a review of the peri-operative care of surgical patients. London: National Confidential Enquiry into Patient Outcome and Death;2011. p. 9.
5. Lim BG, Lee IO. Anesthetic management of geriatric patients. Korean J Anesthesiol. 2020; 73:8–29.

6. Leavitt DA, Motamedinia P, Moran S, Siev M, Zhao PT, Theckumparampil N, et al. Can activities of daily living predict complications following percutaneous nephrolithotomy? J Urol. 2016; 195:1805–9.

7. Brouquet A, Cudennec T, Benoist S, Moulias S, Beauchet A, Penna C, et al. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann Surg. 2010; 251:759–65.

8. Chen TY, Anderson DJ, Chopra T, Choi Y, Schmader KE, Kaye KS. Poor functional status is an independent predictor of surgical site infections due to methicillin-resistant Staphylococcus aureus in older adults. J Am Geriatr Soc. 2010; 58:527–32.
9. Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013; 27:1072–82.

10. Chow B, Rosenthal RA, Merkow RP, Ko CY, Esnaola NF. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg. 2012; 215:453–66.

11. Suskind AM, Walter LC, Jin C, Boscardin J, Sen S, Cooperberg MR, et al. Impact of frailty on complications in patients undergoing common urological procedures: a study from the American College of Surgeons National Surgical Quality Improvement database. BJU Int. 2016; 117:836–42.

12. Isharwal S, Johanning JM, Dwyer JG, Schimid KK, LaGrange CA. Preoperative frailty predicts postoperative complications and mortality in urology patients. World J Urol. 2017; 35:21–6.

13. Sathianathen NJ, Jarosek S, Lawrentschuk N, Bolton D, Konety BR. A simplified frailty index to predict outcomes after radical cystectomy. Eur Urol Focus. 2019; 5:658–63.

14. Mills E, Eyawo O, Lockhart I, Kelly S, Wu P, Ebbert JO. Smoking cessation reduces postoperative complications: a systematic review and meta-analysis. Am J Med. 2011; 124:144–54.

15. Thomsen T, Villebro N, Møller AM. Interventions for preoperative smoking cessation. Cochrane Database Syst Rev. 2014; 27:CD002294.

16. Ferraris VA, Davenport DL, Saha SP, Austin PC, Zwischenberger JB. Surgical outcomes and transfusion of minimal amounts of blood in the operating room. Arch Surg. 2012; 147:49–55.

17. Muñoz M, Acheson AG, Auerbach M, Besser M, Habler O, Kehlet H, et al. International consensus statement on the perioperative management of anaemia and iron deficiency. Anaesthesia. 2017; 72:233–47.

18. Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015; 67:913–24.

19. Chapman E, Pichel AC. Anesthesia for nephrectomy. BJA Education. 2016; 16:98–101.
20. Cassorla L, Lee JW. Patients positioning and associated risks. In: Miller’s Anesthesia. 8th ed. Miller RD, Eriksson LI, Fleisher LA, editors. Philadelphia: Elsevier;2015. p. 1249.
21. Novara G, La Falce S, Kungulli A, Gandaglia G, Ficarra V, Mottrie A. Robot-assisted partial nephrectomy. Int J Surg. 2016; 36:554–9.

22. Cockcroft JO, Berry CB, McGrath JS, Daugherty MO. Anesthesia for major urologic surgery. Anesthesiol Clin. 2015; 33:165–72.

23. Mathuram Thiyagarajan U, Bagul A, Nicholson ML. Pain management in laparoscopic donor nephrectomy: a review. Pain Res Treat. 2012; 2012:201852.
24. Freedland SJ, Blanco-Yarosh M, Sun JC, Hale SJ, Elashoff DA, Litwin MS, et al. Ketorolac-based analgesia improves outcomes for living kidney donors. Transplantation. 2002; 73:741–5.

25. Soulié M, Straub M, Gamé X, Seguin P, De Petriconi R, Plante P, et al. A multicenter study of the morbidity of radical cystectomy in select elderly patients with bladder cancer. J Urol. 2002; 167:1325–8.
26. Knap MM, Lundbeck F, Overgaard J. Early and late treatment-related morbidity following radical cystectomy. Scand J Urol Nephrol. 2004; 38:153–60.

27. Linder BJ, Frank I, Cheville JC, Tollefson MK, Thompson RH, Tarrell RF, et al. The impact of perioperative blood transfusion on cancer recurrence and survival following radical cystectomy. Eur Urol. 2013; 63:839–45.

28. Sui W, Onyeji IC, Matulay JT, James MB, Theofanides MC, Wenske S, et al. Perioperative blood transfusion in radical cystectomy: analysis of the National Surgical Quality Improvement Program database. Int J Urol. 2016; 23:745–50.

29. Abel EJ, Linder BJ, Bauman TM, Bauer RM, Thompson RH, Thapa P, et al. Perioperative blood transfusion and radical cystectomy: does timing of transfusion affect bladder cancer mortality? Eur Urol. 2014; 66:1139–47.

30. Kluth LA, Xylinas E, Rieken M, El Ghouayel M, Sun M, Karakiewicz PI, et al. Impact of peri-operative blood transfusion on the outcomes of patients undergoing radical cystectomy for urothelial carcinoma of the bladder. BJU Int. 2014; 113:393–8.

31. Vetterlein MW, Gild P, Kluth LA, Seisen T, Gierth M, Fritsche HM, et al. Perioperative allogeneic blood transfusion does not adversely affect oncological outcomes after radical cystectomy for urinary bladder cancer: a propensity score-weighted European multicentre study. BJU Int. 2018; 121:101–10.

32. Van der Aa F, Joniau S, Van Den Branden M, Van Poppel H. Metabolic changes after urinary diversion. Adv Urol. 2011; 2011:764325.

33. Amling CL. Diagnosis and management of superficial bladder cancer. Curr Probl Cancer. 2001; 25:219–78.

34. Fredman B, Zohar E, Philipov A, Olsfanger D, Shalev M, Jedeikin R. The induction, maintenance, and recovery characteristics of spinal versus general anesthesia in elderly patients. J Clin Anesth. 1998; 10:623–30.

35. Cesur M, Erdem AF, Alici HA, Yapanoglu T, Yuksek MS, Aksoy Y. The role of succinylcholine in the prevention of the obturator nerve reflex during transurethral resection of bladder tumors. Saudi Med J. 2008; 29:668–71.
36. Koo CH, Chung SH, Kim BG, Min BH, Lee SC, Oh AY, et al. Comparison between the effects of deep and moderate neuromuscular blockade during transurethral resection of bladder tumor on endoscopic surgical condition and recovery profile: a prospective, randomized, and controlled trial. World J Urol. 2019; 37:359–65.

37. Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006; 50:222–7.

38. Cuvas O, Er AE, Ongen E, Basar H. Spinal anesthesia for transurethral resection operations: bupivacaine versus levobupivacaine. Minerva Anestesiol. 2008; 74:697–701.
39. Lee YY, Muchhal K, Chan CK, Cheung AS. Levobupivacaine and fentanyl for spinal anaesthesia: a randomized trial. Eur J Anaesthesiol. 2005; 22:899–903.
40. Bolat D, Aydogdu O, Tekgul ZT, Polat S, Yonguc T, Bozkurt IH, et al. Impact of nerve stimulator-guided obturator nerve block on the short-term outcomes and complications of transurethral resection of bladder tumour: a prospective randomized controlled study. Can Urol Assoc J. 2015; 9:E780–4.

41. Labat G. Regional anesthesia: its technic and clinical application. Philadelphia: WB Saunders Company;1922. p. 249–50.
42. Kakinohana M, Taira Y, Saitoh T, Hasegawa A, Gakiya M, Sugahara K. Interadductor approach to obturator nerve block for transurethral resection procedure: comparison with traditional approach. J Anesth. 2002; 16:123–6.

43. Dagli R, Dadali M, Emir L, Bagbanci S, Ates H. Comparison of classic and inguinal obturator nerve blocks applied for preventing adductor muscle contractions in bladder tumor surgeries: a prospective randomized trial. Urol J. 2019; 16:62–6.
44. Jo YY, Choi E, Kil HK. Comparison of the success rate of inguinal approach with classical pubic approach for obturator nerve block in patients undergoing TURB. Korean J Anesthesiol. 2011; 61:143–7.

45. Choquet O, Capdevila X, Bennourine K, Feugeas JL, Bringuier-Branchereau S, Manelli JC. A new inguinal approach for the obturator nerve block: anatomical and randomized clinical studies. Anesthesiology. 2005; 103:1238–45.
46. Aghamohammadi D, Gargari RM, Fakhari S, Bilehjani E, Poorsadegh S. Classic versus inguinal approach for obturator nerve block in transurethral resection of bladder cancer under spinal anesthesia: a randomized controlled trial. Iran J Med Sci. 2018; 43:75–80.
47. Wassef MR. Interadductor approach to obturator nerve blockade for spastic conditions of adductor thigh muscles. Reg Anesth. 1993; 18:13–7.
48. Hızlı F, Argun G, Güney I, Güven O, Arık AI, Başay S, et al. Obturator nerve block transurethral surgery for bladder cancer: comparison of inguinal and intravesical approaches: prospective randomized trial. Ir J Med Sci. 2016; 185:555–60.
49. Park HP. Irrigation fluids used for transurethral resection of the prostate: a double-edged sword. Korean J Anesthesiol. 2019; 72:87–8.

51. Demirel I, Ozer AB, Bayar M, Erhan OL. TURP syndrome and severe hyponatremia under general anesthesia. BMJ Case Rep. 2012; 2012:bcr-2012-006899.
52. Shin HJ, Lee H, Na HS. The effect of a mixture of 2.7% sorbitol-0.54% mannitol solution on blood coagulation: an in vitro, observational healthy volunteer study using rotational thromboelastometry (ROTEM). Korean J Anesthesiol. 2019; 72:143–9.
53. Shah J, Vyas A, Vyas D. The history of robotics in surgical specialties. Am J Robot Surg. 2014; 1:12–20.

57. Lee JR. Anesthetic considerations for robotic surgery. Korean J Anesthesiol. 2014; 66:3–11.
58. Jo YY, Kwak HJ. What is the proper ventilation strategy during laparoscopic surgery? Korean J Anesthesiol. 2017; 70:596–600.

59. Ahn S, Byun SH, Chang H, Koo YB, Kim JC. Effect of recruitment maneuver on arterial oxygenation in patients undergoing robot-assisted laparoscopic prostatectomy with intraoperative 15 cmH2O positive end expiratory pressure. Korean J Anesthesiol. 2016; 69:592–8.

60. Kim WH, Hahm TS, Kim JA, Sim WS, Choi DH, Lee EK, et al. Prolonged inspiratory time produces better gas exchange in patients undergoing laparoscopic surgery: a randomised trial. Acta Anaesthesiol Scand. 2013; 57:613–22.

61. Lee HJ, Kim KS, Jeong JS, Shim JC, Cho ES. Optimal positive end-expiratory pressure during robot-assisted laparoscopic radical prostatectomy. Korean J Anesthesiol. 2013; 65:244–50.

62. Gupta K, Mehta Y, Sarin Jolly A, Khanna S. Anaesthesia for robotic gynaecological surgery. Anaesth Intensive Care. 2012; 40:614–21.

63. Falabella A, Moore-Jeffries E, Sullivan MJ, Nelson R, Lew M. Cardiac function during steep Trendelenburg position and CO2 pneumoperitoneum for robotic-assisted prostatectomy: a trans-oesophageal Doppler probe study. Int J Med Robot. 2007; 3:312–5.

64. Mavrocordatos P, Bissonnette B, Ravussin P. Effects of neck position and head elevation on intracranial pressure in anaesthetized neurosurgical patients: preliminary results. J Neurosurg Anesthesiol. 2000; 12:10–4.
65. Weber ED, Colyer MH, Lesser RL, Subramanian PS. Posterior ischemic optic neuropathy after minimally invasive prostatectomy. J Neuroophthalmol. 2007; 27:285–7.

66. Awad H, Santilli S, Ohr M, Roth A, Yan W, Fernandez S, et al. The effects of steep trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesth Analg. 2009; 109:473–8.

67. Kalmar AF, Foubert L, Hendrickx JF, Mottrie A, Absalom A, Mortier EP, et al. Influence of steep Trendelenburg position and CO(2) pneumoperitoneum on cardiovascular, cerebrovascular, and respiratory homeostasis during robotic prostatectomy. Br J Anaesth. 2010; 104:433–9.
68. Gainsburg DM. Anesthetic concerns for robotic-assisted laparoscopic radical prostatectomy. Minerva Anestesiol. 2012; 78:596–604.
69. Taylor E, Miller J, Chi T, Stoller ML. Complications associated with percutaneous nephrolithotomy. Transl Androl Urol. 2012; 1:223–8.
70. Malik I, Wadhwa R. Percutaneous nephrolithotomy: current clinical opinions and anesthesiologists perspective. Anesthesiol Res Pract. 2016; 2016:9036872.

71. Hu H, Qin B, He D, Lu Y, Zhao Z, Zhang J, et al. Regional versus general anesthesia for percutaneous nephrolithotomy: a meta-analysis. PLoS One. 2015; 10:e0126587.

72. Srivastava A, Singh KJ, Suri A, Dubey D, Kumar A, Kapoor R, et al. Vascular complications after percutaneous nephrolithotomy: are there any predictive factors? Urology. 2005; 66:38–40.

73. Hosking DH, Bard RJ. Ureteroscopy with intravenous sedation for treatment of distal ureteral calculi: a safe and effective alternative to shock wave lithotripsy. J Urol. 1996; 156:899–901.

74. Chan PS, Fenn J, Li AK. Transurethral ureterorenoscopic lithotripsy and retrieval of ureteric calculi under local anaesthesia and sedation. Br J Urol. 1990; 65:141–3.

75. Vögeli TA, Mellin HE, Hopf B, Ackermann R. Ureteroscopy under local anaesthesia with and without intravenous analgesia. Br J Urol. 1993; 72:161–4.
76. Cybulski PA, Joo H, Honey RJ. Ureteroscopy: anesthetic considerations. Urol Clin North Am. 2004; 31:43–7.

77. Zimmer A, Greul F, Meibner W. Pain management in urology. Urologe A. 2013; 52:585–95.
78. De Oliveira GS, Castro-Alves LJ, Nader A, Kendall MC, McCarthy RJ. Transversus abdominis plane block to ameliorate postoperative pain outcomes after laparoscopic surgery: a meta-analysis of randomized controlled trials. Anesth Analg. 2014; 118:454–63.
79. Aniskevich S, Taner CB, Perry DK, Robards CB, Porter SB, Thomas CS, et al. Ultrasound-guided transversus abdominis plane blocks for patients undergoing laparoscopic hand-assisted nephrectomy: a randomized, placebo-controlled trial. Local Reg Anesth. 2014; 7:11–6.

80. Baeriswyl M, Zeiter F, Piubellini D, Kirkham KR, Albrecht E. The analgesic efficacy of transverse abdominis plane block versus epidural analgesia: a systematic review with meta-analysis. Medicine (Baltimore). 2018; 97:e11261.
81. Cacciamani GE, Menestrina N, Pirozzi M, Tafuri A, Corsi P, De Marchi D, et al. Impact of combination of local anesthetic wounds infiltration and ultrasound transversus abdominal plane block in patients undergoing robot-assisted radical prostatectomy: perioperative results of a double-blind randomized controlled trial. J Endourol. 2019; 33:295–301.

82. Dal Moro F, Aiello L, Pavarin P, Zattoni F. Ultrasound-guided transversus abdominis plane block (US-TAPb) for robot-assisted radical prostatectomy: a novel ‘4-point’ technique-results of a prospective, randomized study. J Robot Surg. 2019; 13:147–51.

83. Güner Can M, Göz R, Berber İ, Kaspar Ç, Çakır Ü. Ultrasound/laparoscopic camera-guided transversus abdominis plane block for renal transplant donors: a randomized controlled trial. Ann Transplant. 2015; 20:418–23.

84. Hosgood SA, Thiyagarajan UM, Nicholson HF, Jeyapalan I, Nicholson ML. Randomized clinical trial of transversus abdominis plane block versus placebo control in live-donor nephrectomy. Transplantation. 2012; 94:520–5.

85. Parikh BK, Waghmare VT, Shah VR, Mehta T, Butala BP, Parikh GP, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane block for retroperitoneoscopic donor nephrectomy: a randomized controlled study. Saudi J Anaesth. 2013; 7:43–7.

86. Matulewicz RS, Patel M, Jordan BJ, Morano J, Frainey B, Bhanji Y, et al. Transversus abdominis plane blockade as part of a multimodal postoperative analgesia plan in patients undergoing radical cystectomy. Bladder Cancer. 2018; 4:161–7.

87. Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997; 78:606–17.

88. Vukovic N, Dinic L. Enhanced recovery after surgery protocols in major urologic surgery. Front Med (Lausanne). 2018; 5:93.

89. Bai Y, Wang X, Li X, Pu C, Yuan H, Tang Y, et al. Management of catheter-related bladder discomfort in patients who underwent elective surgery. J Endourol. 2015; 29:640–9.

90. Yu D, Chai W, Sun X, Yao L. Emergence agitation in adults: risk factors in 2,000 patients. Can J Anaesth. 2010; 57:843–8.

91. Park M, Jee CH, Kwak KH, Park JM, Kim JH. The effect of preoperative nefopam treatment on postoperative catheter-related bladder discomfort in patients undergoing transurethral bladder tumor resection: a randomized double-blind study. Scand J Urol. 2018; 52:389–94.

92. Cheon YW, Kim SH, Paek JH, Kim JA, Lee YK, Min JH, et al. Effects of nefopam on catheter-related bladder discomfort in patients undergoing ureteroscopic litholapaxy. Korean J Anesthesiol. 2018; 71:201–6.

93. Jendoubi A, Aissi W, Abbes A, Bouzouita A, Fourati S, Necib H, et al. Efficacy and safety of parecoxib for prevention of catheter-related bladder discomfort in patients undergoing transurethral resection of bladder tumor: prospective randomised trial. Indian J Anaesth. 2018; 62:461–5.
94. Kwon SY, Joo JD, Cheon GY, Oh HS, In JH. Effects of dexmedetomidine infusion on the recovery profiles of patients undergoing transurethral resection. J Korean Med Sci. 2016; 31:125–30.

95. Stanfa LC, Singh L, Williams RG, Dickenson AH. Gabapentin, ineffective in normal rats, markedly reduces C-fibre evoked responses after inflammation. Neuroreport. 1997; 8:587–90.

96. Yoshimura N, Chancellor MB. Current and future pharmacological treatment for overactive bladder. J Urol. 2002; 168:1897–913.

97. Bala I, Bharti N, Chaubey VK, Mandal AK. Efficacy of gabapentin for prevention of postoperative catheter-related bladder discomfort in patients undergoing transurethral resection of bladder tumor. Urology. 2012; 79:853–7.

98. Kim JA, Min JH, Lee HS, Jo HR, Je UJ, Paek JH. Effects of glycopyrrolate premedication on preventing postoperative catheter-related bladder discomfort in patients receiving ureteroscopic removal of ureter stone. Korean J Anesthesiol. 2016; 69:563–7.

99. Gray M, Vasdev N, Gowrie-Mohan S, McNicholas T. The management of unplanned erection during endoscopic urological surgery. Curr Urol. 2017; 10:113–7.

100. Miller PD, Galizia EJ. Management of erections during transurethral surgery using ethyl chloride spray. Br J Urol. 1993; 71:105.

101. Seftel AD, Resnick MI, Boswell MV. Dorsal nerve block for management of intraoperative penile erection. J Urol. 1994; 151:394–5.

102. Jang D, Lim CS, Shin YS, Ko YK, Park SI, Song SH, et al. A comparison of regional and general anesthesia effects on 5 year survival and cancer recurrence after transurethral resection of the bladder tumor: a retrospective analysis. BMC Anesthesiol. 2016; 16:16.

103. Choi WJ, Baek S, Joo EY, Yoon SH, Kim E, Hong B, et al. Comparison of the effect of spinal anesthesia and general anesthesia on 5-year tumor recurrence rates after transurethral resection of bladder tumors. Oncotarget. 2017; 8:87667–74.

104. Koumpan Y, Jaeger M, Mizubuti GB, Tanzola R, Jain K, Hosier G, et al. Spinal anesthesia is associated with lower recurrence rates after resection of nonmuscle invasive bladder cancer. J Urol. 2018; 199:940–6.

105. Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012; 130:1237–50.

106. Hole A, Unsgaard G, Breivik H. Monocyte functions are depressed during and after surgery under general anaesthesia but not under epidural anaesthesia. Acta Anaesthesiol Scand. 1982; 26:301–7.

107. Markovic SN, Knight PR, Murasko DM. Inhibition of interferon stimulation of natural killer cell activity in mice anesthetized with halothane or isoflurane. Anesthesiology. 1993; 78:700–6.

108. Wada H, Seki S, Takahashi T, Kawarabayashi N, Higuchi H, Habu Y, et al. Combined spinal and general anesthesia attenuates liver metastasis by preserving TH1/TH2 cytokine balance. Anesthesiology. 2007; 106:499–506.

109. Piegeler T, Votta-Velis EG, Liu G, Place AT, Schwartz DE, Beck-Schimmer B, et al. Antimetastatic potential of amide-linked local anesthetics: inhibition of lung adenocarcinoma cell migration and inflammatory Src signaling independent of sodium channel blockade. Anesthesiology. 2012; 117:548–59.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download