This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Several hospitals have implemented a multidisciplinary Acute Pain Service (APS) to execute surgery-specific opioid sparing analgesic pathways. Implementation of an anesthesia attending-only APS has been associated with decreased postoperative opioid consumption, time to ambulation, and time to solid food intake for patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. In this study, we evaluated the impact of introducing an APS trainee on postoperative opioid consumption in patients undergoing hyperthermic intraperitoneal chemotherapy during postoperative day (POD) 0–3.
Methods
We performed a retrospective propensity-matched cohort study where we compared opioid consumption and hospital length of stay among two historical cohorts: attending-only APS service versus service involving a regional anesthesia fellow.
Results
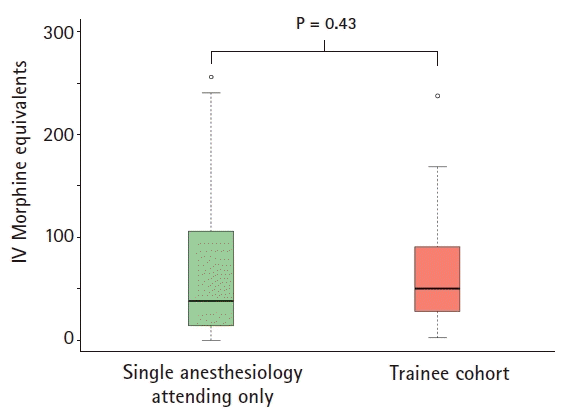
In the matched cohorts, POD 0–3 opioid use [25%, 75% quartile] for the single attending and trainee involvement cohort were 38.5 mg morphine equivalents (MEQ) [14.1 mg, 106.3 mg] and 50.4 mg MEQ [28.4 mg, 91.2 mg], respectively. The median difference was –9.8 mg MEQ (95% CI –30.7 to 16.5 mg; P = 0.43). There was no difference in hospital length of stay between both cohorts (P = 0.67).
Conclusions
We found that the addition of a regional anesthesia fellow to the APS team was not associated with statistically significant differences in total opioid consumption or hospital length of stay in this surgical population. The addition of trainees to the infrastructure, with vigilant supervision, is not associated with change in outcomes.
Keywords: Acute pain service, Epidural, Opioids, Trainee
Introduction
Inadequate postsurgical pain control has been associated with adverse events including myocardial ischemia, ileus, pulmonary infections, anxiety, thromboembolism, as well as chronic persistent postoperative pain [
1]. Similarly, patients with persistently poorly controlled pain throughout their admission are more likely to have 30-day readmission or subsequent emergency department visits [
2]. Reliance on unimodal opioid therapy for pain management has resulted in serious side effects [
3] leading to the evolution of multimodal analgesia as part of the practice guidelines for perioperative pain management [
4]. Several hospitals have implemented a multidisciplinary Acute Pain Service (APS) to execute these surgery-specific opioid sparing analgesic pathways.
The implementation of an anesthesia attending-only APS has been associated with decreased postoperative opioid consumption, time to ambulation, and time to solid food intake for patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) [
5]. In September 2017, the APS at our institution introduced a regional anesthesia fellow into the APS team. In this propensity-matched cohort study, we evaluated the impact of introducing an APS trainee on postoperative opioid consumption in patients undergoing CRS-HIPEC during postoperative day (POD) 0–3.
Materials and Methods
Data was collected retrospectively from the data warehouse of our institution. All data for surgical patients that were scheduled for a CRS-HIPEC from 2016 to 2018 was extracted. The resulting dataset remained de-identified and did not contain sensitive patient-health information as defined by the institutional Human Research Protections Program, and therefore, was exempt from the informed consent requirement and approved by our University of California, San Diego Institutional Review Board (UCSD IRB no. 190559).
This was a retrospective cohort study in which the primary objective was to determine if there was a decrease in median total opioid consumption (intravenous morphine equivalents [MEQ]) during POD 0–3 among CRS-HIPEC patients whose pain was managed by a single anesthesiology attending provider versus a team consisting of an anesthesiology attending and a trainee (regional anesthesia and acute pain fellow) in the setting of APS. During 2016 to mid-2017, APS consisted of a single anesthesiology attending provider fellowship-trained in either regional anesthesia and acute pain or chronic pain. Thereafter, a weekly rotating trainee (regional anesthesia and acute pain fellow) was introduced into the APS team. The multimodal analgesic regimen implemented by APS includes preoperative pregabalin, scopolamine patch, and pre-operative thoracic epidural placement in addition to around-the-clock dosing of acetaminophen. This protocol has not changed with the addition of trainees to the APS model. We sought to determine if there was an association of trainee involvement in opioid consumption.
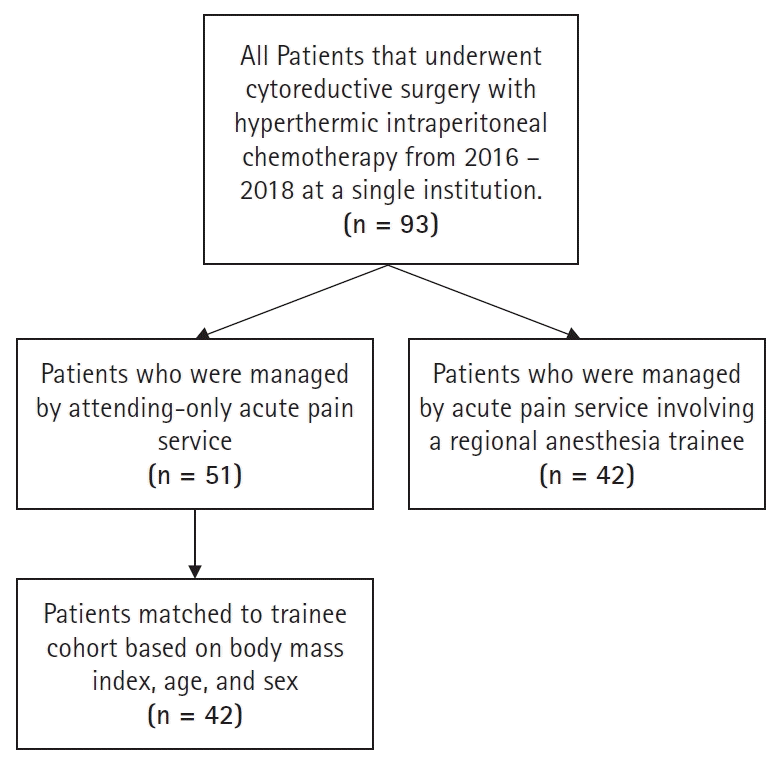
Fig. 1 illustrates patient inclusion criteria. We performed a propensity-matched (controlling for age, body mass index, sex, and primary cancer site) comparison. Between matched cohorts, we compared median opioid consumption during POD 0–3 using Wilcoxon rank sum test. An absolute standardized mean difference of less than 0.2 between cohorts among each confounder variable was considered adequately matched. The median difference and 95% CI were calculated using the Hodges-Lehman estimator. A P < 0.05 was considered statistically significant. The manuscript adheres to the applicable Equator guidelines.
Results
There were a total of 51 patients managed by a single anesthesiology attending versus 42 patients in the trainee involvement cohort. In the unmatched cohorts, the median POD 0–3 opioid use [25%, 75% quartile] for the single anesthesiology attending versus the trainee involvement cohort were 27.5 mg MEQ [7.6 mg, 106.25 mg] versus 50.4 mg MEQ [28.4 mg, 91.2 mg], respectively, with a median difference of –15.2 mg MEQ (95% CI 33.8–5.40 mg MEQ, P = 0.14). Demographic variables between the propensity-matched cohorts all had a standardized mean difference < 0.2, and therefore were considered appropriately matched (
Table 1). In the matched cohorts, the median POD 0–3 opioid use [25%, 75% quartile] for the single anesthesiology attending and trainee involvement cohort were 38.5 mg MEQ [14.1 mg, 106.3 mg] and 50.4 mg MEQ [28.4 mg, 91.2 mg], respectively. The median difference was –9.8 mg MEQ (95% CI –30.7 to 16.5 mg; P = 0.43) (
Fig. 2). There was no difference in hospital length of stay between both cohorts (P = 0.67).
Discussion
We found no statistically significant differences in total opioid consumption on POD 0–3 in patients undergoing CRP-HIPEC when the APS team consisted of an anesthesia attending and trainee, compared to a single anesthesiology attending. Likewise, there was no statistically significant difference in hospital length of stay between the two groups. The success of APS in reducing opioid requirement is multifactorial, and includes implementation of multimodal analgesic regimens, patient education, close follow-up, and successful pre-operative placement of thoracic epidural analgesia (TEA). The addition of trainees to the infrastructure, with vigilant supervision, is not associated with change in outcomes. The multimodal analgesic regimen implemented by APS includes preoperative pregabalin, scopolamine patch, and pre-operative thoracic epidural placement in addition to around-the-clock dosing of acetaminophen [
5]. This protocol has not changed with the addition of trainees to the APS model. Likewise, every epidural placed in the pre-operative holding area was tested with ice, and a bilateral sensory level to ice confirmed before going to the operating room [
5]. Epidural catheters that did not have a bilateral sensory level to ice were removed, thus only patients with epidural catheters that had a positive bilateral sensory level loss to ice were included in this study.
As Tran and Krodel [
6] pointed out, logistics about optimal organization of an APS is a question that has yet to be resolved. Infrastructure varies widely between institutions, some incorporating attendings, residents, and fellows in Anesthesiology only, while others are multidisciplinary integrating pharmacists, psychologists, and physiotherapists into their infrastructure [
7]. As Tran and Krodel discusses, there are short-term upsides to single anesthesiology attending only APS, including assurance in block success in terms of neuraxial procedure as well as vigilance in patient follow-up and optimization of established protocols. However, an infrastructure of a one-person service is non-sustainable and does not allow for growth. Likewise, in an academic institution, such infrastructure can limit opportunities for valuable educational experiences in multimodal analgesia, as well as one-on-one expert instruction in TEA placement for trainees.
TEA procedure continues to be a challenging procedure for anesthesiologists with failure rates as high as 32% in large teaching institutions [
8,
9]. Unfortunately, opportunities for trainees to learn TEA seem to be decreasing [
10] due to a variety of reasons including falsely perceived increased risk of complications, inexperienced supervisory role, and introduction of alternative blocks such as transverse abdominis plane blocks [
8]. At UCSD, it is not surprising that the quality of outcomes has not changed with the addition of trainees given the established setup of APS workflow: 1) early discussion of the analgesia plan with the surgical team, 2) efficient workflow with patient and nursing check-in, 3) supervising the attending specialized in regional anesthesia and highly experienced in TEA placement, and 4) ample time allowed to test block success defined by sensory deficit. Even with trainees involved, such organization allows for such system to be setup for success.
Limitations of this study include its retrospective design, which may involve unmeasured confounders. Likewise, preoperative and intraoperative opioid data was not collected, which could affect postoperative opioid requirement. Although our APS manages a variety of surgical populations, we chose to focus on CRS-HIPECs to maintain consistency in the population and because they tend to use the highest opioids among major surgery. Furthermore, we may be under-powered to detect any real differences. A larger prospective randomized controlled trial will be required to answer this question definitively.
In conclusion, including trainees to APS infrastructure is not associated with change in patient outcomes in terms of postoperative opioid requirement or hospital length of stay.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download