Introduction
Materials and Methods
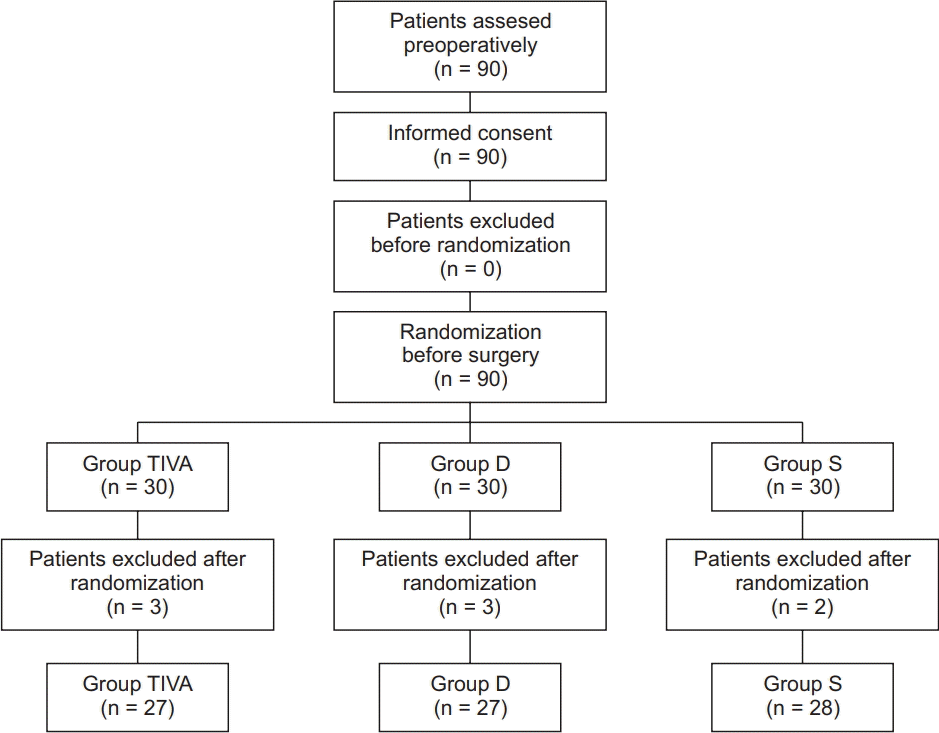
Study design
Participants
Randomization
Blinding
Interventions
Outcomes
Statistical analysis and sample size
Results
Table 1.
| Group TIVA (n = 27) | Group D (n = 27) | Group S (n = 28) | P value | |
|---|---|---|---|---|
| ASA (I/II)† | 8/19 | 13/14 | 6/22 | 0.115 |
| Gender (M/F)† | 15/12 | 12/15 | 12/16 | 0.641 |
| Age (yr)‡ | 43.1 ± 13.0 | 46.5 ± 13.1 | 45.2 ± 12.5 | 0.615 |
| Height (cm)§ | 168.7 ± 9.2 | 166.6 ± 9.1 | 168.0 ± 6.9 | 0.494 |
| Weight (kg)‡ | 85.4 ± 16.4 | 80.5 ± 16.7 | 84.6 ± 16.4 | 0.510 |
| BMI (kg/m2)‡ | 29.8 ± 5.0 | 28.5 ± 6.0 | 29.8 ± 5.7 | 0.633 |
| Duration of surgery (min)§,|| | 180.0 ± 16.9 | 195.1 ± 8.4 | 173.1 ± 5.9 | < 0.001* |
Table 2.
| Group TIVA (n = 27) | Group D (n = 27) | Group S (n = 28) | P value | |
|---|---|---|---|---|
| FEV1 (%) | 0.336* | |||
| Baseline | 81.6 ± 19.0 | 83.4 ± 18.9 | 73.4 ± 17.8 | |
| 30th min | 63.8 ± 22.0 | 67.5 ± 20.3 | 60.40 ± 22.3 | |
| 24 h | 71.4 ± 22.8 | 69.03 ± 17.5 | 67.96 ± 16.9 | |
| P value† | < 0.001† | < 0.001† | < 0.001† | |
| FVC (%) | 0.031* | |||
| Baseline | 84.0 ± 21.4 | 88.6 ± 27.4 | 71.9 ± 18.7 | |
| 30th min | 65.6 ± 20.4 | 73.5 ± 26.3 | 62.2 ± 22.2 | |
| 24 h | 74.0 ± 22.9 | 69.4 ± 14.8 | 66.7 ± 17.1 | |
| P value† | < 0.001† | < 0.001† | 0.002† | |
| FEV1/FVC | 0.104* | |||
| Baseline | 102.0 ± 16.3 | 99.0 ± 19.0 | 107.9 ± 16.2 | |
| 30th min | 101.7 ± 15.6 | 101.5 ± 17.7 | 100.9 ± 14.1 | |
| 24th h | 100.0 ± 15.1 | 105.4 ± 17.7 | 105.6 ± 11.7 | |
| P value† | 0.815 | 0.243 | 0.009† |
Values are presented as mean ± SD. FEV1: Forced expiratory volume in the 1st second, FVC: Forced vital capacity.
Table 3.
| Group TIVA (n = 27) | Group D (n = 27) | Group S (n = 28) | P value | |
|---|---|---|---|---|
| PaO2 (mmHg) | 0.299* | |||
| Baseline | 100.5 ± 19.4 | 94.7 ± 16.4 | 91.7 ± 13.7 | |
| After intubation | 140.4 ± 41.3 | 151.5 ± 53.8 | 143.5 ± 32.8 | |
| Before extubation | 153.9 ± 38.6 | 159.2 ± 40.6 | 160.8 ± 29.3 | |
| 30th min | 121.0 ± 35.0 | 126.2 ± 38.8 | 132.1 ± 46.2 | |
| 24 h | 93.7 ± 20.9 | 83.7 ± 16.8 | 84.6 ± 16.1 | |
| P value† | < 0.001† | < 0.001† | < 0.001† | |
| PaCO2 (mmHg) | 0.843* | |||
| Baseline | 34.1 ± 5.0 | 34.0 ± 3.6 | 35.8 ± 3.1 | |
| After intubation | 35.3 ± 3.2 | 34.9 ± 3.6 | 35.4 ± 4.7 | |
| Before extubation | 37.1 ± 2.6 | 36.4 ± 2.7 | 37.1 ± 3.2 | |
| 30th min | 42.0 ± 4.0 | 40.9 ± 3.4 | 42.5 ± 3.4 | |
| 24 h | 35.3 ± 4.5 | 35.4 ± 3.8 | 35.4 ± 3.5 | |
| P value† | < 0.001† | < 0.001† | < 0.001† | |
| pH | 0.632* | |||
| Baseline | 7.4 ± 0.04 | 7.4 ± 0.02 | 7.4 ± 0.03 | |
| After intubation | 7.4 ± 0.03 | 7.4 ± 0,04 | 7.4 ± 0.05 | |
| Before extubation | 7.4 ± 0.03 | 7.4 ± 0.03 | 7.3 ± 0.04 | |
| 30th min | 7.3 ± 0.03 | 7.3 ± 0.03 | 7.3 ± 0.04 | |
| 24 h | 7.4 ± 0.04 | 7.4 ± 0.04 | 7.4 ± 0.03 | |
| P value† | < 0.001† | < 0.001† | < 0.001† | |
| SaO2 (%) | 0.736* | |||
| Baseline | 97.7 ± 1.2 | 97.5 ± 1.2 | 97.5 ± 1.0 | |
| After intubation | 98.6 ± 0.7 | 98.2 ± 1.5 | 98.8 ± 0.5 | |
| Before extubation | 98.8 ± 0.3 | 98.4 ± 0.8 | 98.9 ± 0.6 | |
| 30th min | 97.8 ± 1,6 | 98.0 ± 1.4 | 98.1 ± 1.6 | |
| 24 h | 97.2 ± 1.8 | 96.9 ± 1.6 | 97.0 ± 1.6 | |
| P value† | < 0.001† | < 0.001† | < 0.001† | |
| HCO3− (mmol/L) | 0.685* | |||
| Baseline | 24.0 ± 2.0 | 23.5 ± 1.3 | 23.2 ± 1.3 | |
| After intubation | 24.4 ± 1.4 | 23.5 ± 1.4 | 23.2 ± 2.2 | |
| Before extubation | 24.2 ± 1.3 | 23.4 ± 1.6 | 23.3 ± 1.8 | |
| 30th min | 24.1 ± 1.5 | 23.1 ± 1.3 | 23.3 ± 1.9 | |
| 24 h | 25.3 ± 1.6 | 24.6 ± 1.7 | 24.9 ± 1.9 | |
| P value† | < 0.001† | < 0.001† | < 0.001† | |
| BE (mmol/L) | 0.698* | |||
| Baseline | −0.7 ± 2.2 | −1.4 ± 1.5 | −1.4 ± 1.4 | |
| After intubation | −0.3 ± 1.6 | −1.3 ± 1.6 | −1.5 ± 2.3 | |
| Before extubation | −0.3 ± 1.4 | −1.3 ± 1.7 | −1.4 ± 2.0 | |
| 30th min | −0,4 ± 1,8 | −1,2 ± 1,5 | −1,1 ± 2,2 | |
| 24 h | 0.7 ± 1.9 | 0.07 ± 1.9 | 0.5 ± 2.2 | |
| P value† | < 0.001† | < 0.001† | < 0.001† | |
| Lactate (mmol/L) | 0.027* | |||
| Baseline | 1.5 ± 0.6 | 1.1 ± 0.4 | 1.4 ± 0.6 | |
| After intubation | 1.5 ± 0.5 | 1.2 ± 0.5 | 1.5 ± 0.6 | |
| Before extubation | 1.4 ± 0.6 | 1.2 ± 0.5 | 1.7 ± 0.9 | |
| 30th min | 1.4 ± 0.5 | 1.4 ± 0.6 | 1.8 ± 0.9 | |
| 24 h | 1.5 ± 0.6 | 1.4 ± 0.8 | 1.3 ± 0.6 | |
| P value† | 0.723 | 0.051 | 0.012† | |
| PaO2/FiO2 | 0.268* | |||
| Baseline | 457.4 ± 78.7 | 443.0 ± 90.7 | 437.1 ± 65.1 | |
| After intubation | 349.4 ± 101.1 | 376.6 ± 138.1 | 358.2 ± 82.4 | |
| Before extubation | 380.9 ± 100.2 | 390.4 ± 111.10 | 401.4 ± 73.6 | |
| 30th min | 322.5 ± 81.9 | 326.9 ± 99.8 | 339.7 ± 113.9 | |
| 24th h | 425.0 ± 87.8 | 378.4 ± 83.9 | 392.7 ± 67.8 | |
| P value† | < 0.001† | < 0.001† | < 0.001† |
Values are presented as mean ± SD. PaO2: Partial pressure of arterial oxygen, PaCO2: Partial pressure of arterial carbon dioxide, pH: Potential of hydrogen, SaO2: Arterial oxygen saturation, [HCO3−]: Bicarbonate, BE: Base excess, PaO2/FiO2: Partial pressure of arterial oxygen/fraction of inspired oxygen.
* comparison among the groups, Repeated Measures of ANOVA. There was no difference in PaO2, PaCO2, pH, SaO2, [HCO−3], base excess, lactate, and PaO2/FiO2 values among the groups (P = 0.299, 0.843, 0.632, 0.736, 0.685, 0.698, 0.027, and 0.268, respectively) (P < 0.016 indicates statistically significant difference among the groups).
Table 4.
| Group TIVA (n = 27) | Group D (n = 27) | Group S (n = 28) | P value | |
|---|---|---|---|---|
| C (ml/cmH2O) | 0.176* | |||
| After intubation‡ | 62.7 ± 19.4 | 66.7 ± 19.3 | 58.9 ± 15.8 | |
| 1st h of operation‡ | 57.5 ± 13.4 | 62.2 ± 19.5 | 54.0 ± 14.0 | |
| 2nd h of operation‡ | 53.8 ± 11.7 | 51.8 ± 11.0 | 47.4 ± 9.2 | |
| P value† | < 0.001† | < 0.001† | < 0.001† | |
| Raw (cm/H2O/L/s) | 0.697* | |||
| After intubation‡ | 9.8 ± 5.4 | 9.0 ± 1.7 | 9.4 ± 2.5 | |
| 1st h of operation‡ | 9.5 ± 2.4 | 9.1 ± 2.0 | 9.9 ± 2.7 | |
| 2nd h of operation‡ | 9.3 ± 2.2 | 8.9 ± 1.3 | 9.8 ± 1.6 | |
| P value† | 0.808 | 0.778 | 0.372 | |
| Cumulative Morphine consumption§,∥ (mg) | 7.1 ± 1.1 | 6.7 ± 1.1 | 7.5 ± 1.0 | 0.030* |
∥ : Bonferoni corrected Mann Whitney U. There is no difference in dynamic lung compliance, airway resistance and cumulative morphine consumption among the groups (P = 0.176, 0.679 and 0.030 respectively) (P < 0.016 indicates statistically significant difference among the groups).
† : inter-group comparisons, repeated measures ANOVA. The dynamic lung compliance reduced at the 1st and 2nd h of operation than after intubation in all groups (P < 0.001 for all). No difference in airway resistance at any measurement intervals within the groups (P = 0.808 in the Group TIVA, P = 0.778 in the Group D, P = 0.372 in the Group S) (P < 0.05 indicates statistically significant difference within group comparisons).




 Citation
Citation Print
Print



 XML Download
XML Download