Introduction
Materials and Methods
Results
Effects of BMS-470539 on the secretion of pro-inflammatory cytokines by LPS-stimulated neutrophils
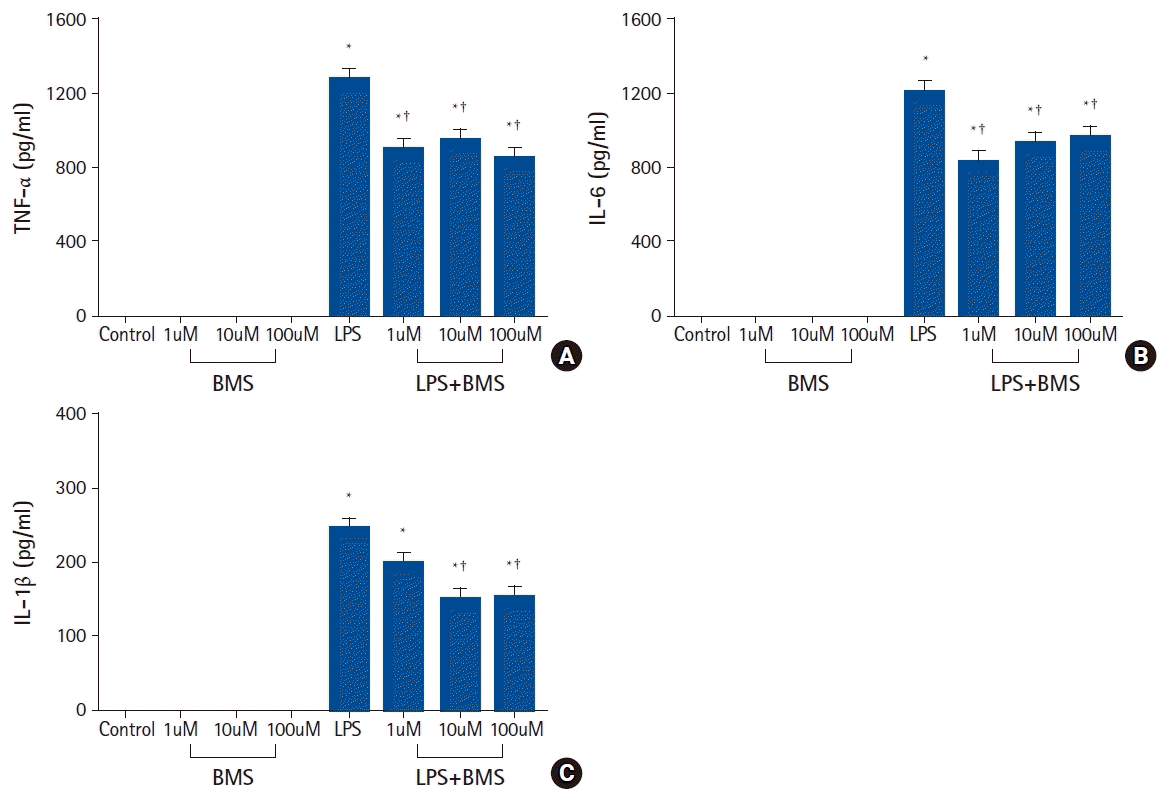 | Fig. 1.The effects of BMS-470539 on pro-inflammatory cytokine (TNF-α, IL-6, and IL-1β) production in human neutrophil stimulated by lipopolysaccharide (LPS).
Neutrophils (5 × 106/ml) from human blood were incubated for 4 h without or with BMS-470539 (1 µM, 10 µM, and 100 µM), or with LPS (100 ng/ml) or LPS plus BMS-470539 (Control, BMS, LPS, LPS + BMS, respectively). The level of cytokine (A) TNF-α, (B) IL-6, and (C) IL-1β were obtained from ELISA. Values are presented as mean ± SD (n = 6 per group). *P < 0.05 versus control, †P < 0.05 versus LPS.
|
Effects of BMS-470539 on the MAPKs and NF-κB activation in LPS-stimulated neutrophils
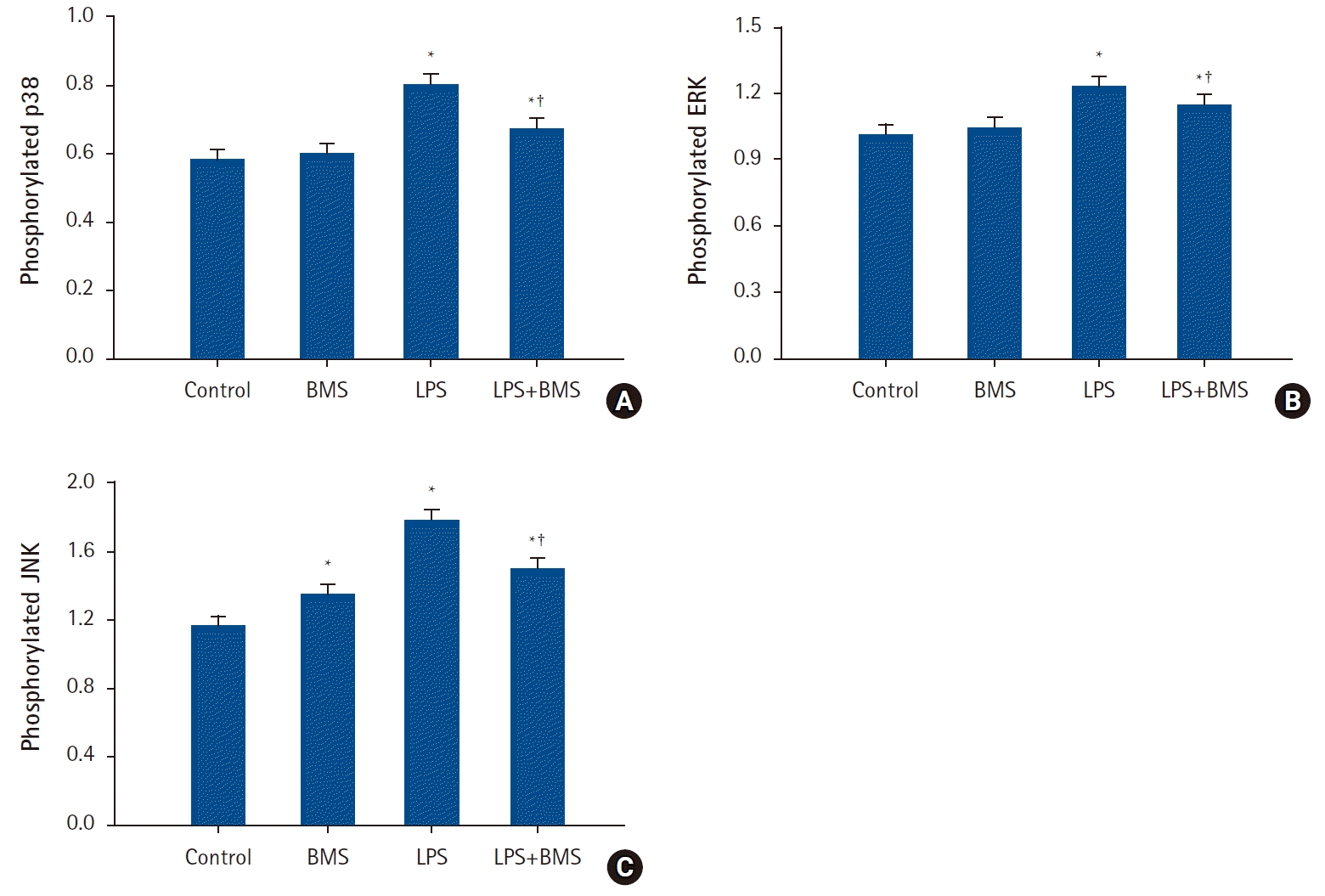 | Fig. 2.The effects of BMS-470539 on mitogen-activated protein kinases activation in human neutrophil stimulated by lipopolysaccharide (LPS).
Neutrophils (5 × 106/ml) from human blood were incubated for 30 min without or with BMS-470539 (100 µM), or with LPS (100 ng/ml) or LPS plus BMS-470539 (Control, BMS, LPS, LPS + BMS, respectively). The phosphorylation levels of (A) p38, (B) ERK, and (C) JNK were measured by ELISA. Values are presented as mean ± SD (n = 4 per group). *P < 0.05 versus Control, †P < 0.05 versus LPS.
|
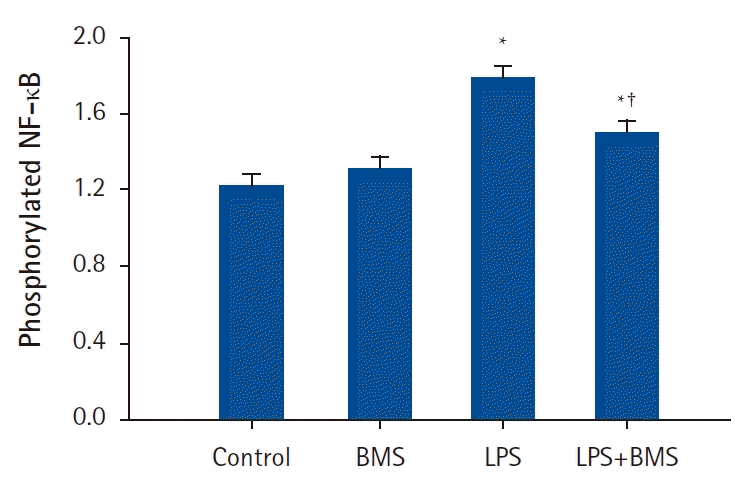 | Fig. 3.The effects of BMS-470539 on nuclear translocation of nuclear factor kappa B (NF-κB) in human neutrophils stimulated by lipopolysaccharide (LPS).
Neutrophils (5 × 106/ml) from human blood were incubated for 30 min without or with BMS-470539 (100 µM), or with LPS (100 ng/ml) or LPS plus BMS-470539 (Control, BMS, LPS, LPS + BMS, respectively). The phosphorylation level of NF-κB was measured by ELISA. Values are presented as mean ± SD (n = 4 per group). *P < 0.05 versus with Control, †P < 0.05 versus LPS.
|
Effect of BMS-470539 on LPS-delayed neutrophil apoptosis
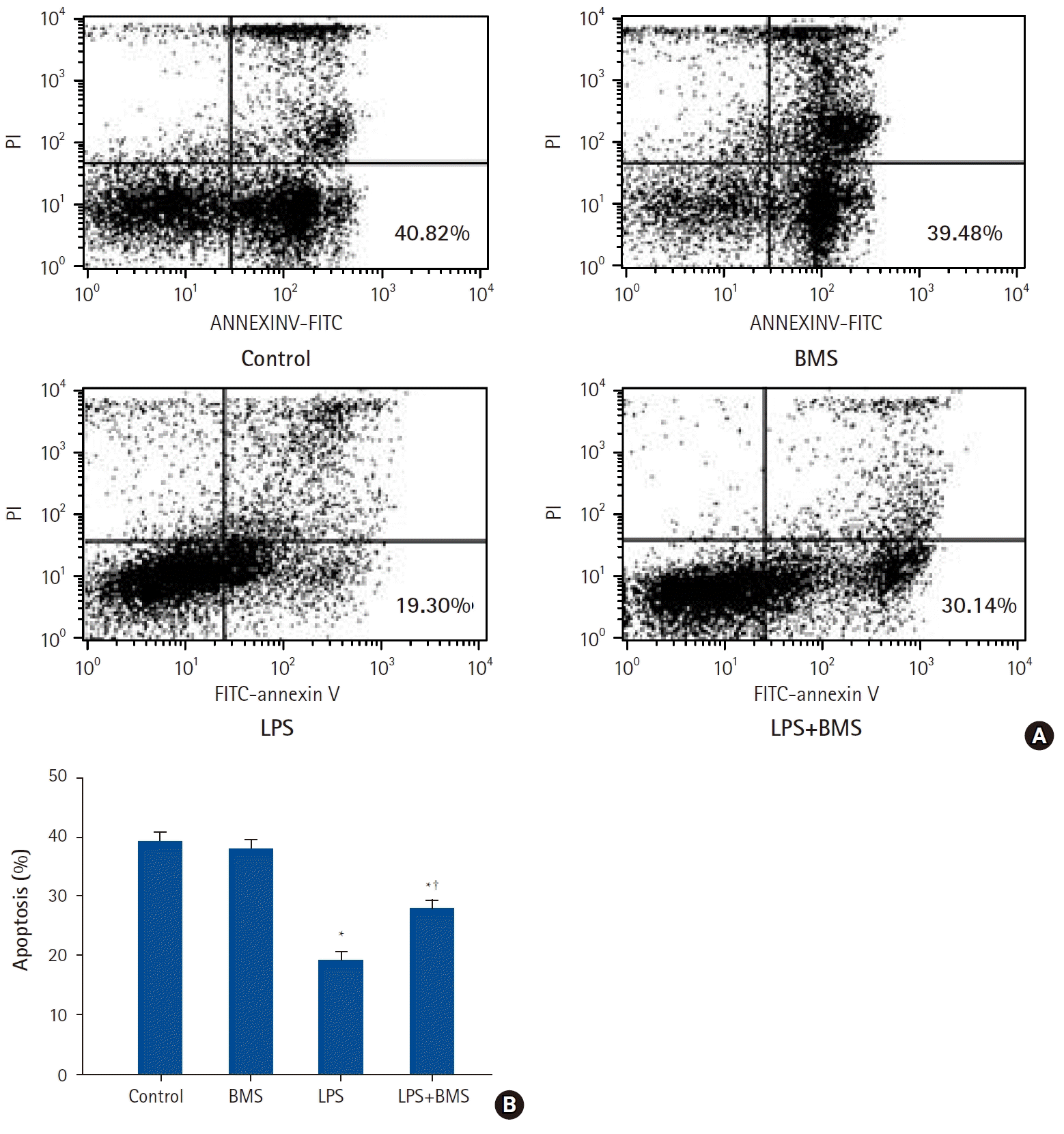 | Fig. 4.The effects of BMS-470539 on apoptosis of human neutrophils stimulated by lipopolysaccharide (LPS).
Neutrophils (5 × 106/ml) from human blood were incubated for 24 h without or with BMS-470539 (100 µM), or with LPS (100 ng/ml) or LPS plus BMS-470539 (Control, BMS, LPS, LPS + BMS, respectively). (A) Contour diagram of fluorescein isothiocyanate annexin V/propidium iodide (FITC-annexin V/PI) flow cytometry of neutrophils for different groups. The lower right quadrants represent the apoptotic cells, FITC-annexin V positive and PI negative. One representative experiment out of six is shown. (B) The percentage of neutrophil apoptosis was determined for each group. Data are shown as mean ± SD (n = 6 per group). *P < 0.05 versus Control, †P < 0.05 versus LPS.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download